About Cross Reporting
Automated cross reporting extends the evaluation of reporting obligations to include cross-reporting (investigational to marketing registration) scenarios.
Vault Safety evaluates the investigational and marketing registrations of study products, then identifies any additional reporting destinations for products and studies. Administrators can also specify destination overrides on product licenses.
Once this feature is enabled, see Cross Reporting to learn more.
Enable Investigational to Marketing Same Agency Cross Reporting
You must rename, and then add the Investigational to Marketing field to the Agency page layout to allow Business Administrators to enable the field for agencies that require Investigational to Marketing same-agency cross reporting.
Go to Configuration > Objects > Organization and make the following updates:
- From the Fields tab, rename the Inv. to Marketing (same agency) field to "Investigational to Marketing"
- From the Page Layouts tab, update the Agency Page Layout to add the Investigational to Marketing field to the Details section.
- To enable FDA IND-to-NDA cross reporting, go the FDA Agency record, and enable the Investigational to Marketing field.
Enable Configurable Transmission Profile Selection
The Transmission Profile field allows admins to set the Transmission Profile to use for a Study or Product Registration. Additionally, the Transmission Profile Scope object was introduced for the cross reporting feature, and allows admins to configure criteria to match Transmission Profiles, if a Study or Product Registration doesn’t specify one.
Make the following page layout updates to allow Business Administrators to configure the Transmission Profile for Submission rules.
Manage page layouts by going to Configuration > Objects, opening the object, and going to the Page Layouts tab of the relevant object.
| Object | Page Layout | Changes |
|---|---|---|
| Transmission Profile | All (AS2, Email, Manual, System, Base) | Add a Transmission Profile Scope related object section:
|
| Transmission Profile Scope | Transmission Profile Scope Page Layout | Ensure the Details section contains the following fields:
|
| Study Registration | Study Registration Page Layout | Add the following fields to the Details section:
|
| Product Registration | Product Registration Page Layout | Add the following field to the Details section:
|
Make Product Registration Field Optional for Study Products
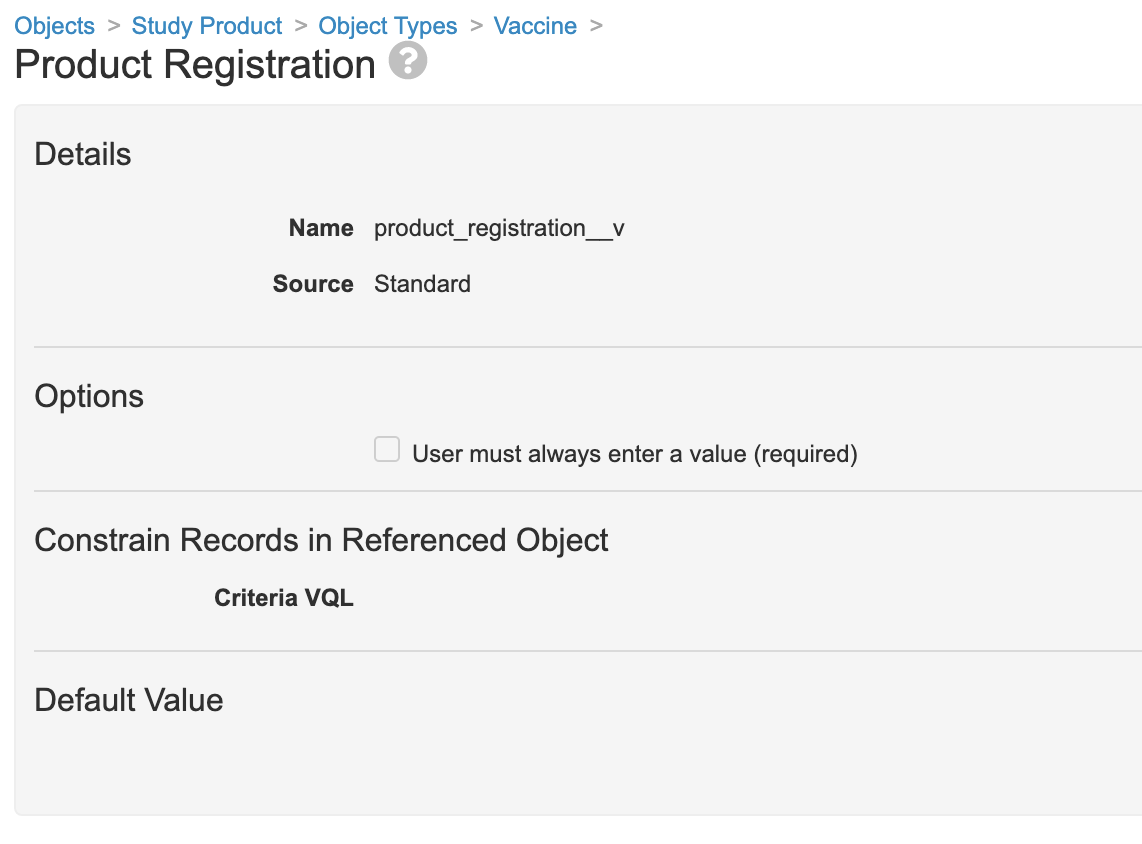
You can make the Product Registration field optional on the Study Product object.
- Go to Configuration > Objects > Study Product > Object Types.
- In all object types, open the Product Registration field, and then deselect USer must always enter a value (required).
Next Steps
Once your Vault has the configuration described on this page, the following list describes next steps to turn on cross reporting in your Vault:
- To turn on cross reporting in your Vault, you must enable the Cross-Reporting application setting.
- To enable cross reporting for the FDA Rule Set, you must update the Active Version of the FDA ICSR Reporting Rule Set to
3. Manage Reporting Rule Versions provides more information.
