About the Feature
Vault Safety now supports Case processing for the following new Product Types: OTC Drugs, Cosmetics, and Nutritionals. You can create new Product records using these Product Types. In addition, Products can now include multiple Product Registrations for different Product Types to support agency-specific reporting requirements.
For details on setting up Products with the new Product types, see Manage Products.
Update the Product Detail Page Layout for Product and Product Type
- Go to Admin > Configuration > Objects > Product > Page Layouts.
- Select the Product Detail Page Layout.
- In the Registrations section, select Edit Columns and add the Product and Product Type fields.
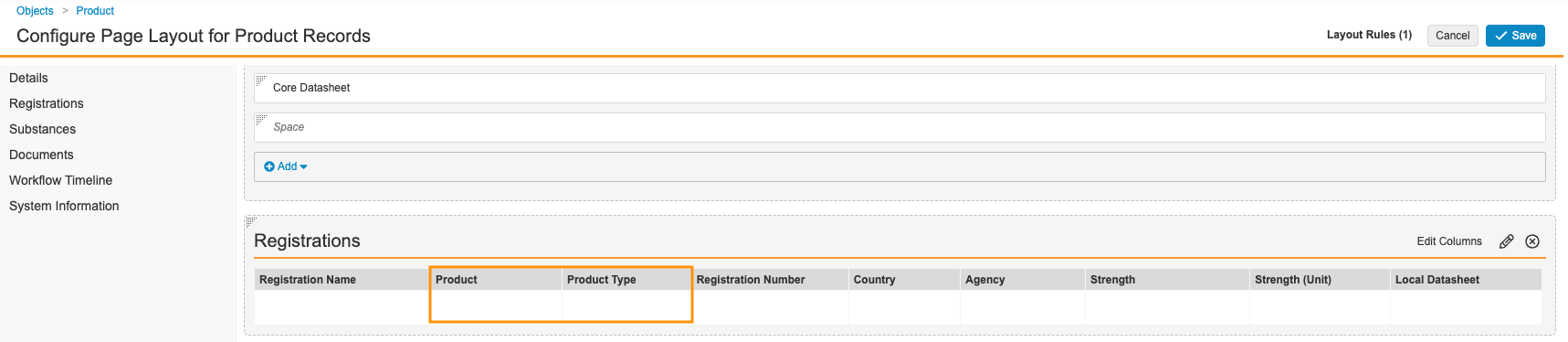
Product Detail Page Layout with Product and Product Type - Save the updated layout.
Add the Case Product Company Product Detail Page Layout
- Go to Admin > Configuration > Objects > Case Product > Page Layouts.
- Select Create.
- On the Add Page Layout page, select Company Product from the Object Type picklist, and then select Create.
- On the Configure Page Layout for Company Product Records page, configure the sections and fields as shown below. To rearrange sections and fields, drag and drop.
Note Some fields are field controls, which are denoted by a (
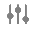 ) symbol. These are also shown in the screenshot below.
) symbol. These are also shown in the screenshot below.
Note In addition to the fields shown above, the Globally Registered As field is available for administrator use. This field lists all of the Transmission Product Types across all Product Registrations for a Product. To avoid end users editing the field, keeping the Globally Registered As field hidden is recommended.
Update the Case Product Related Object to Include Company Product
- Go to Admin > Configuration > Objects > Case > Page Layouts.
- Select the Case Page Layout.
- On the right side of the Products section, select the pencil icon.

Edit Case Product Related Object - On the Edit Related Object Section page, update the Criteria VQL field to include company product with the following VQL:
object_type__vr.api_name__v CONTAINS
('device__v', 'biologic__v', 'drug__v', 'external__v', 'company_product__v')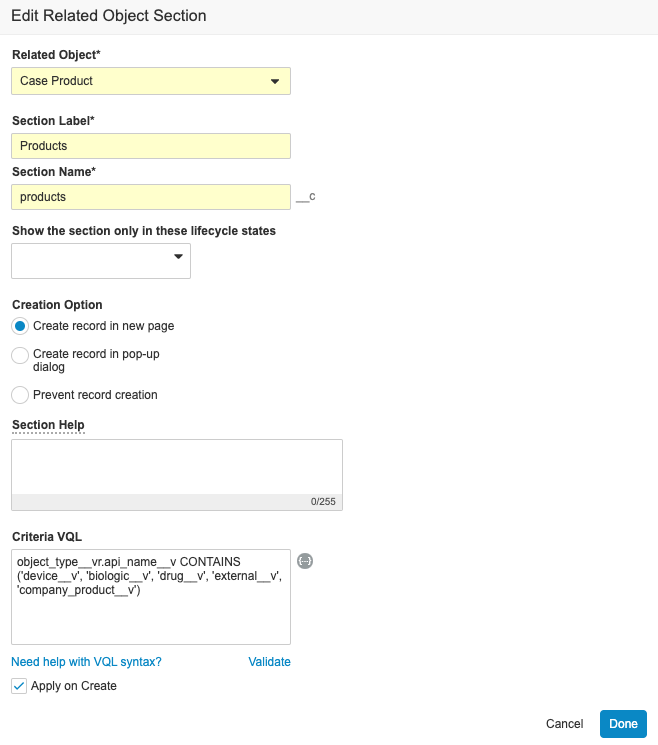
Edit Case Product Related Object VQL - Select Done
- On the Configure Page Layout for Case Records page, select Save.
Note If your organization wants to use the Company Product product type when adding Study Products to Studies, contact Veeva Support.
