About the Feature
Vault Safety can now track substances and their use in individual products. When adding a Case Product that is associated with a pre-configured Substance, the substance information is snapshotted to the Case Product.
This feature was added to Vault Safety in 20R2 and is available in vaults deployed in 20R2 or later by default. For vaults deployed in 20R1 or earlier, an administrator must perform the following configuration changes to enable this feature.
Manage Products and Manage Studies provide more information about this feature.
Update Permission Sets for Object Access
As an Administrator, we recommend that you update the relevant permission sets for the following objects to comply with new object configurations. We also recommend that you enable the permission to Read Substance and Product Substance objects for all users. For users who will be managing Products, you should enable the permission to Edit Substance and Product Substance objects.
- In the Admin area, go to Users & Groups > Permission Sets, and then select the permission set you want to update.
- In the permission set, select Objects, and then select Edit.
- For the respective objects, select the applicable checkboxes to grant permissions to the following objects.
- Substance
- Case Product Substance
- Product Substance
- Reporting Family Member Type
- Select Save.
Result
Your permission set has been updated with your selected object permissions.
Configure the Product and Drug Page Layout to Add Related Object Section for Substances
- In the Admin area, go to Configuration > Objects > Product > Page Layouts, and then select Product Detail Page Layout.
- Select Add Section at the top of the page, and then select Related Object. A box will appear.
- Fill in the specified fields:
- Select Save at the top of the page.
- Repeat the above steps for Drug Page Layout.
Result
Your Product and Drug records are now configured to have the Related Object section for Substances.
Note You can optionally add the Substances section to the Biologics Page Layout depending on your business requirements.
Configure the Substance Page Layout to Add Related Objects Section for Products
- In the Admin area, go to Configuration > Objects > Substance > Page Layouts, and then select Substance Detail Page Layout.
- Select Add Section at the top of the page, and then select Related Object. A box will appear.
- Fill in the specified fields:
- Select Save at the top of the page.
Result
Your Substance records are now configured to have the Related Object section for Product Substance.
Configure the Case Product Substance Page Layout to Add Strength (Number) and Strength (Unit) Fields
- In the Admin area, go to Configuration > Objects > Case Product Substance > Page Layouts, and then select Case Product Substance Detail Page Layout.
- There are two options available to add these fields
- In the Details section, select Add, and then select
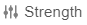 . This is the combined Strength control that combines the Strength (Number) and Strength (Unit) fields together.
. This is the combined Strength control that combines the Strength (Number) and Strength (Unit) fields together.
Note We recommend you use this option.
- In the Substance Details section, select Add, and then select Strength (Number) and Strength (Unit).
- In the Details section, select Add, and then select
- Select Save at the top of the page.
Result
Your Case Product Substance records are now configured to display the Strength field(s).
Configure the Study Product Substance Page Layout to Include the New Substance Field
- In the Admin area, go to Configuration > Objects > Study Product Substance > Page Layouts, and then select Study Product Substance Detail Page Layout.
- In the Details section, select Add, and then select Substance Name.
- Select Save.
Result
Your Study Product Substance records are now configured to display the Substance field.
Verify the Case Product Page Layout Includes Substances
Most vaults include the Substances section on the Case Product Page Layout by default. However, some vaults based on older templates may not have this configuration.
Go to Configuration > Objects > Case Product > Page Layouts, and verify that there is a Substances related objects section in the following Case Product page layouts:
- Biologic Page Layout
- Case Product Detail Page Layout
- Drug Page Layout
- External Product Page Layout
- Study Product Page Layout
If the section is not present, add a related object section for Substances.
(Optional) Remove Study Product Substance from Page Layouts
This configuration is optional but highly recommended. Update this configuration on the following page layouts:
- Biologic Page Layout
- Drug Page Layout
- External Product Page Layout
- Study Product Detail Page Layout
- In the Admin area, go to Configuration > Objects > Study Product > Page Layouts, and then open the page layout.
- Remove the Substances related object section:
Remove Substances Related Object Section - Save the page layout, and repeat these steps for each of the Study Product page layouts mentioned above.
Result
The Substance section has been deleted from your Study Product records.
(Optional) Remove Study Product Substance from Page Layouts
You can optionally update your vault’s Business Admin (Quick Access) navigation tab to add Substances as a subtab for convenient navigation. To add a subtab, go to Configuration > Vault Navigation > Tabs.
