About the Feature
Vault Safety 20R3 introduced support for vaccine product types and data capture for VAERS submissions.
The following list outlines the configuration required to make this feature available for vaults, depending on the version the vault was originally deployed from:
- 20R2 and Earlier: Follow all of the instructions on this page to enable vaccines in vaults originally deployed in 20R2 or an earlier release.
- 20R3 and Later: The 20R3 Vault Safety template includes most of the configuration required to use this feature by default. However, your vault may require updates to the Transmission object lifecycle. Review the steps outlined under Update the Transmission Lifecycle.
- All Versions: To display VAERS related dosage fields for external vaccines, the External Product Type field must be added to the External Case Product Page Layout.
Once this feature is enabled, Prepare Vaccine Submissions provides instructions for users.
Note For FDA VAERS electronic submissions, you must also configure the FDA Gateway.
New in 21R1: Add External Product Type Field to External Product Page Layout
To display VAERS related dosage fields for external vaccines, the External Product Type field must be added to the External Case Product Page Layout. This field is not displayed on page layouts by default.
Go to Configuration > Objects > Case Product > External Product Page Layout, and add the External Product Type field to the layout.
Ensure to update the appropriate Permission Sets to grant access to Edit this field.
Vaccine Combination Products
You must perform additional configuration to use combination products with a vaccine constituent. The combination product enablement instructions provide more information.
Activate Vaccine Object Type
Activate the Vaccine object type for the following objects:
- Case Product
- Product
- Study Product
Activate VAERS Transmission Profile
In Business Admin > Transmission Profiles, move the CBER VAERS Transmission Profile to the Active state.
Note You may need to add a user action to Change State to Active on the Transmission Profile lifecycle to trigger the state change.
Activate Controlled Vocabularies
In Business Admin > Controlled Vocabularies, move the following Qualification-type Controlled Vocabularies to the Active state:
- Patient
- Parent
Update Navigation Tabs
In Configuration > Tabs, make the following changes to the navigation tabs:
- Under Business Admin (Quick Access), delete the Product Library tab.
- Add a new Product Library tab under Business Admin (Quick Access) with the following settings:
- Type: Product
- Object Type: Vaccines, Biologics, Drugs, Devices, Combination Products
Update the Transmission Lifecycle
Go to Configuration > Object Lifecycles > Transmission Lifecycle.
Pending State
Make the following changes to the Pending state:
- Entry Criteria: Add CBER VAERS and GWTest to the FDA ESG Entry Criteria that validates the Transmission Profile when the Submission Method equals FDA ESG.
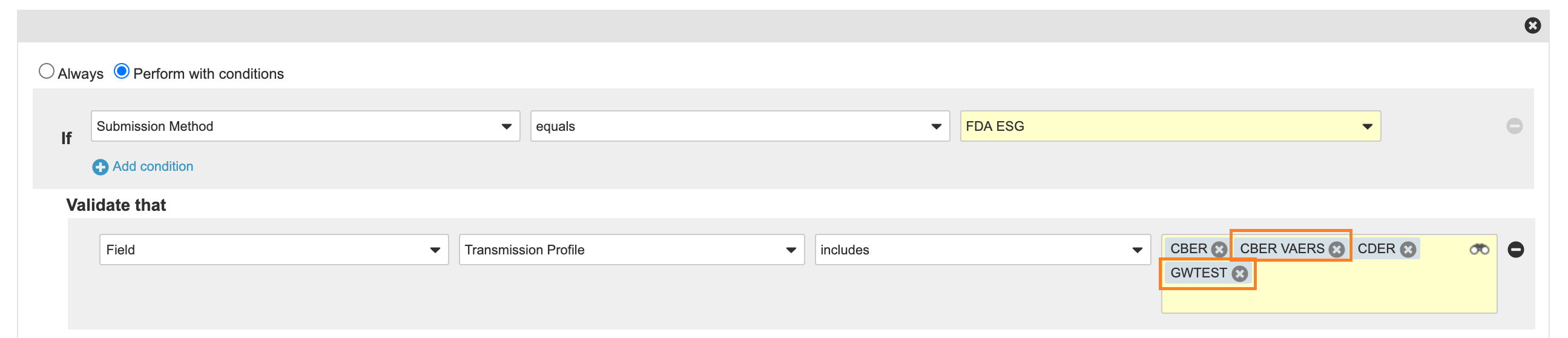
New Pending State Entry Criteria - Entry Action: Add CBER VAERS to the CBER, CDER Entry Action to start the Submission: FDA ESG Workflow:
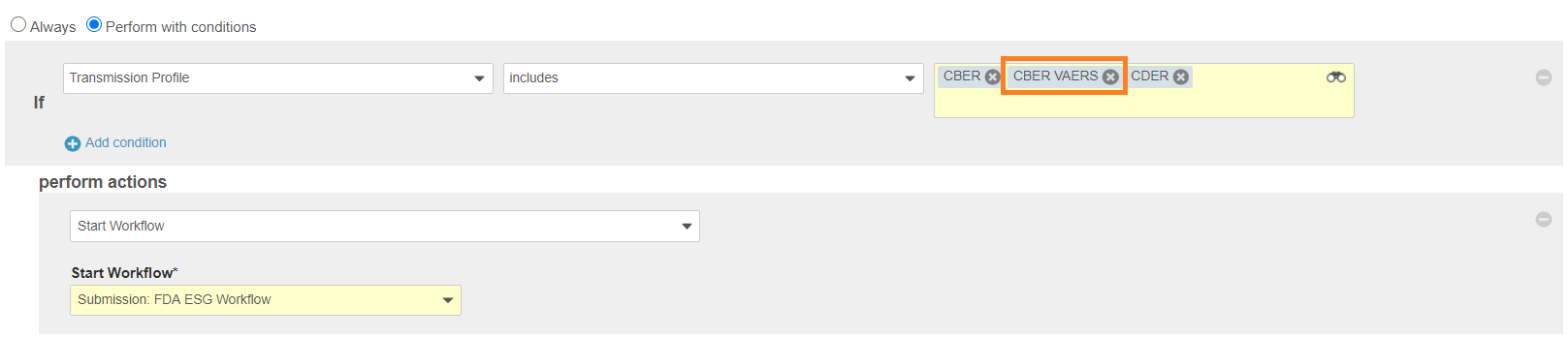
New Pending State Entry Action
Ready for Submission State
Make the following changes to the Ready for Submission state:
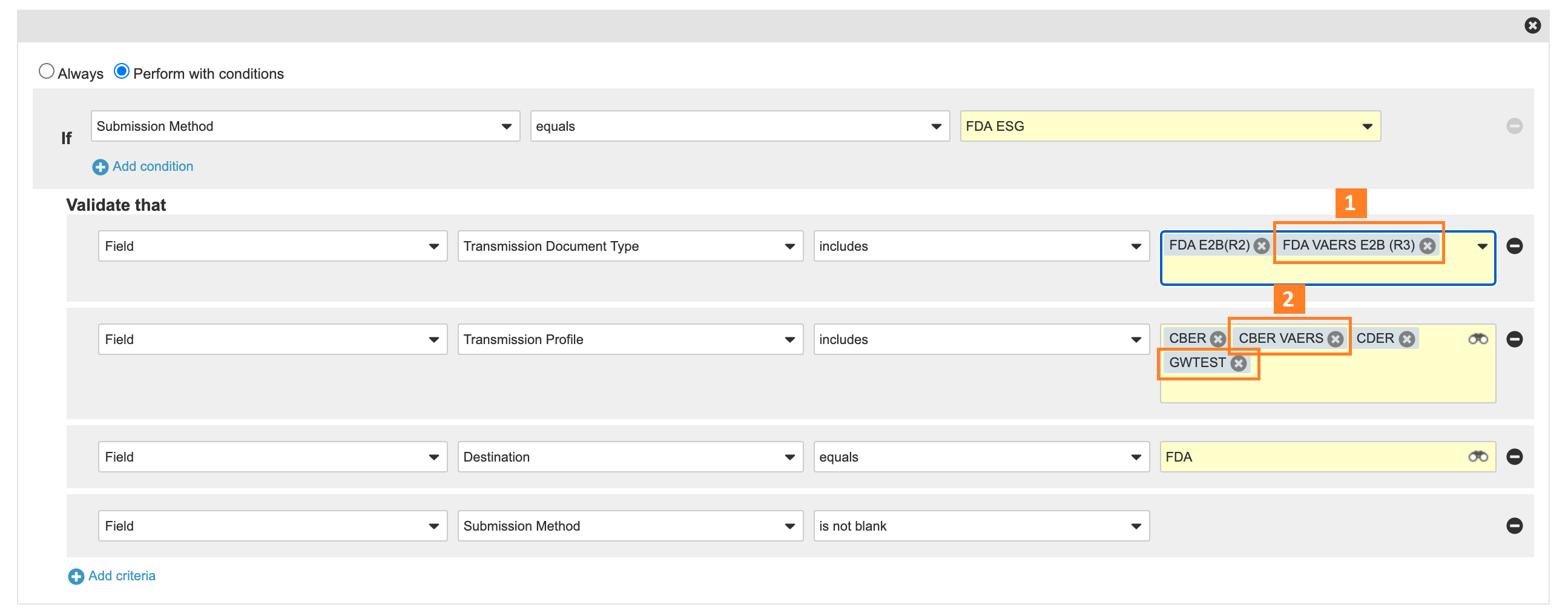
Entry Criteria:
- Add FDA VAERS E2B (R3) to the FDA ESG Entry Criteria that validates the Transmission Document Type.
- Add CBER VAERS and GWTest to the FDA ESG Entry Criteria that validates the Transmission Profile.
Configure Page Layouts
Manage page layouts from the Page Layouts tab on the appropriate object from Configuration > Objects.
Case Page Layouts
Update page layouts for the following Case object types:
- AER
- Case
- Imported Case
- Parental Case
The following table outlines the sections that require updates and the relevant page layouts for each change:
| Page Layouts | Section | Changes |
|---|---|---|
|
Products | Edit the Products section to include the Vaccine object type. |
|
Reporters | Make the following changes to the Reporters section:
|
|
Patient | Make the following changes to the Case Patient section:
|
|
Patient | Add the following combined field controls to the Patient section:
|
|
Parent | Add the Age at Vaccination field. |
(Optional) Case Page Layout Rules
The following page layout rules are optional but recommended for user experience.
| Page Layouts | Rule Label | HIDE THE FOLLOWING PAGE LAYOUT ITEMS | IF THIS LAYOUT RULE EXPRESSION IS TRUE |
|---|---|---|---|
|
Reporter Fields |
|
reporter_qualification__vr.name__v = 'Patient' |
|
Not Female | Update the existing Not Female (not_female__c) layout rule to hide the following items:
|
gender_value__vr.name__v != "Female" (unchanged) |
|
Hide if not Vaccine |
|
product_type__vr.name__v != "Vaccine" |
Case Contact Page Layouts
Update the Reporter-type Case Contact page layout and edit the page layouts for the Facility, Patient, and Health Care Professional object types.
Reporter Detail Page Layout
Make the following changes to the Reporter Detail Page Layout:
- Replace the Email field with the Email combined field control
- Add the Street Line 2 field.
- Add the County combined field control.
- Add the Best Doctor? field.
Facility Detail Page Layout
See the following table for the Facility Detail Page Layout setup:
| Section | Fields |
|---|---|
| Details |
|
| Address |
|
| Workflow Timeline | Default Settings |
| System Information | Default Settings |
Patient Contact Detail Page Layout
See the following table for the Patient Contact Detail Page Layout setup:
| Section | Fields |
|---|---|
| Details |
|
| Address |
|
| Workflow Timeline | Default Settings |
| System Information | Default Settings |
Health Care Professional Detail Page Layout
See the following table for the Health Care Professional Detail Page Layout setup:
| Section | Fields |
|---|---|
| Details |
|
| Workflow Timeline | Default Settings |
| System Information | Default Settings |
Case Product Page Layout
Add the Vaccine Type field.
Product Page Layout
Add the Vaccine Type field.
Study Product Page Layout
Add the Vaccine object type to the Study Products section.
Case Adverse Event Page Layout
Add a Hospitalization/Treatment section with the following settings:
- Label: Hospitalization/Treatment
- Layout: Detail Form - One Column
- Fields:
- Hospitalization
- Evaluated/Treated At
- Days Hospitalized
- Hospital Name
- Hospital City
- Hospital State
- Hospital Admission Date
- Hospital Discharge Date
Case Medical History Page Layout
Add the Illness at Vaccination field.
Case Drug History Page Layout
Add the Age at Vaccination Control
Study Page Layout
Add the Vaccine object type on the Study Product related object section.
Update Permissions
- Administrator Actions > Tabs: Add View access to Product Library tab.
- Permission Sets: Update permission sets according to the settings described in the following table.
| Permission Set | Object | Object Type | Object Permissions | Field | Field Permissions | Action | Action Permissions |
|---|
| Permission Set | Object | Object Type | Permission | Field | Permission | Action | Permission | |
|---|---|---|---|---|---|---|---|---|
| Administration Actions | Case Contact | Facility | Read | All Fields | Read | - | - | |
| Administration Actions | Case Contact | Patient Contact | Read | All Fields | Read | - | - | |
| Administration Actions | Case Contact | Health Care Professional | Read | All Fields | Read | - | - | |
| Administration Actions | Case Product | Vaccine | Read | All Fields | Read | - | - | |
| Administration Actions | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Administration Actions | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Administration Actions | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Administration Actions | Product | Vaccine | Create, Read, Update, Delete | All Fields | Read | - | - | |
| Administration Actions | Study Product | Vaccine | Create, Read, Update, Delete | All Fields | Read | - | - | |
| Administration Actions | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Administration Actions | Validation Result | - | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Case Contact | Facility | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Case Contact | Patient Contact | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Case Contact | Health Care Professional | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Case Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Case Distribution Actions | Validation Result | - | Read | All Fields | Read | - | - | |
| Case Entry Actions | Case | - | - | Reporter Language | Edit | - | - | |
| Case Entry Actions | Case | - | - | Reporter Email Address | Edit | - | - | |
| Case Entry Actions | Case | - | - | Reporter Email Consent | Edit | - | - | |
| Case Entry Actions | Case | - | - | Age at Vaccination (number) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Age At Vaccination (unit) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Age At Vaccination (normalized) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Pregnant at Exposure | Edit | - | - | |
| Case Entry Actions | Case | - | - | Pregnant at Exposure (Reason Omitted) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient Name Prefix | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient Name Prefix (Reason Omitted) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient First Name | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient First Name (Reason Omitted) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient Middle Name | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient Middle Name (Reason Omitted) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient Last Name | Edit | - | - | |
| Case Entry Actions | Case | - | - | Patient Last Name (Reason Omitted) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Race (Reason Omitted) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Ethnicity (Reason Omitted) | Edit | - | - | |
| Case Entry Actions | Case | - | - | Military Status | Edit | - | - | |
| Case Entry Actions | Case | - | - | Pregnancy Conception Date | Edit | - | - | |
| Case Entry Actions | Case | - | - | Pregnancy Due Date | Edit | - | - | |
| Case Entry Actions | Case | - | - | Reason Received Late | Edit | - | - | |
| Case Entry Actions | Case | - | - | External System UID | Edit | - | - | |
| Case Entry Actions | Case | - | - | Suppress Submission | Edit | - | - | |
| Case Entry Actions | Case Contact | Facility | Create, Read, Update, Delete | - | - | - | - | |
| Case Entry Actions | Case Contact | Patient Contact | Create, Read, Update, Delete | - | - | - | - | |
| Case Entry Actions | Case Contact | Health Care Professional | Create, Read, Update, Delete | - | - | - | - | |
| Case Entry Actions | Case Product | Vaccine | Create, Read, Update, Delete | All Fields | Edit | - | - | |
| Case Entry Actions | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Case Entry Actions | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Case Entry Actions | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Case Entry Actions | Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Entry Actions | Study Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Entry Actions | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Case Entry Actions | Validation Result | - | Read | All Fields | Read | - | - | |
| Case Intake Actions | Case | - | - | Reporter Language | Edit | - | - | |
| Case Intake Actions | Case | - | - | Reporter Email Address | Edit | - | - | |
| Case Intake Actions | Case | - | - | Reporter Email Consent | Edit | - | - | |
| Case Intake Actions | Case | - | - | Age at Vaccination (number) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Age At Vaccination (unit) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Age At Vaccination (normalized) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Pregnant at Exposure | Edit | - | - | |
| Case Intake Actions | Case | - | - | Pregnant at Exposure (Reason Omitted) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient Name Prefix | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient Name Prefix (Reason Omitted) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient First Name | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient First Name (Reason Omitted) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient Middle Name | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient Middle Name (Reason Omitted) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient Last Name | Edit | - | - | |
| Case Intake Actions | Case | - | - | Patient Last Name (Reason Omitted) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Race (Reason Omitted) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Ethnicity (Reason Omitted) | Edit | - | - | |
| Case Intake Actions | Case | - | - | Military Status | Edit | - | - | |
| Case Intake Actions | Case | - | - | Pregnancy Conception Date | Edit | - | - | |
| Case Intake Actions | Case | - | - | Pregnancy Due Date | Edit | - | - | |
| Case Intake Actions | Case | - | - | Reason Received Late | Edit | - | - | |
| Case Intake Actions | Case | - | - | External System UID | Edit | - | - | |
| Case Intake Actions | Case | - | - | Suppress Submission | Edit | - | - | |
| Case Intake Actions | Case Contact | Facility | Read | All Fields | Read | - | - | |
| Case Intake Actions | Case Contact | Patient Contact | Read | All Fields | Read | - | - | |
| Case Intake Actions | Case Contact | Health Care Professional | Read | All Fields | Read | - | - | |
| Case Intake Actions | Case Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Intake Actions | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Case Intake Actions | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Case Intake Actions | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Case Intake Actions | Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Intake Actions | Study Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Intake Actions | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Case Intake Actions | Validation Result | - | Read | All Fields | Read | - | - | |
| Case Review Actions | Case Contact | Facility | Read | All Fields | Read | - | - | |
| Case Review Actions | Case Contact | Patient Contact | Read | All Fields | Read | - | - | |
| Case Review Actions | Case Contact | Health Care Professional | Read | All Fields | Read | - | - | |
| Case Review Actions | Case Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Review Actions | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Case Review Actions | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Case Review Actions | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Case Review Actions | Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Review Actions | Study Product | Vaccine | Read | All Fields | Read | - | - | |
| Case Review Actions | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Case Review Actions | Validation Result | - | Read | All Fields | Read | - | - | |
| Case Translation Actions | Case | - | - | - | - | Evaluate Regulatory Conformance | No Access | |
| Lock Manager Actions | Case | - | - | - | - | Evaluate Regulatory Conformance | No Access | |
| Safety Operations Actions | Case Contact | Facility | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Case Contact | Patient Contact | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Case Contact | Health Care Professional | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Case Product | Vaccines | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Product | Vaccine | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Study Product | Vaccine | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Safety Operations Actions | Validation Result | - | Read | All Fields | Read | - | - | |
| Safety Operations Actions | - | - | - | - | - | All Actions | No Access | |
| Safety Writer | Case Contact | Facility | Read | All Fields | Read | - | - | |
| Safety Writer | Case Contact | Patient Contact | Read | All Fields | Read | - | - | |
| Safety Writer | Case Contact | Health Care Professional | Read | All Fields | Read | - | - | |
| Safety Writer | Case Product | Vaccine | Read | All Fields | Read | - | - | |
| Safety Writer | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Safety Writer | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Safety Writer | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Safety Writer | Product | Vaccine | Read | All Fields | Read | - | - | |
| Safety Writer | Study Product | Vaccine | Read | All Fields | Read | - | - | |
| Safety Writer | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Safety Writer | Validation Result | - | Read | All Fields | Read | - | - | |
| Safety Writer | - | - | - | - | - | Lock Case | No Access | |
| Safety Writer | - | - | - | - | - | Unlock Case | No Access | |
| Safety Writer | - | - | - | - | - | Evaluate Regulatory Conformance | No Access | |
| Safety Writer | - | - | - | - | - | - | - | |
| Submission Actions | Case Contact | Facility | Read | All Fields | Read | - | - | |
| Submission Actions | Case Contact | Patient Contact | Read | All Fields | Read | - | - | |
| Submission Actions | Case Contact | Health Care Professional | Read | All Fields | Read | - | - | |
| Submission Actions | Case Product | Vaccine | Read | All Fields | Read | - | - | |
| Submission Actions | Controlled Vocabulary | Anatomical Site | Read | All Fields | Read | - | - | |
| Submission Actions | Controlled Vocabulary | Facility Type | Read | All Fields | Read | - | - | |
| Submission Actions | Controlled Vocabulary | Military Status Type | Read | All Fields | Read | - | - | |
| Submission Actions | Controlled Vocabulary | Ethnicity | Read | - | - | - | - | |
| Submission Actions | Product | Vaccine | Read | All Fields | Read | - | - | |
| Submission Actions | Study Product | Vaccine | Read | All Fields | Read | - | - | |
| Submission Actions | Validation Criteria | - | Read | All Fields | Read | - | - | |
| Submission Actions | Validation Result | - | Read | All Fields | Read | - | - |
