Sections in This Article
About Safety Rules
The Safety Rules in a Safety Rule Set define the conditions for which a Transmission is generated. Each Safety Rule has a set of parameters. The parameters must evaluate successfully for the rule to pass.
A Safety Rule’s Priority defines the order in which the system attempts to match each reporting rule, which are processed from lowest to highest. To prevent over-reporting, once the system finds the first matching rule, further rules are not evaluated.
The following image shows an example of a Safety Rule:
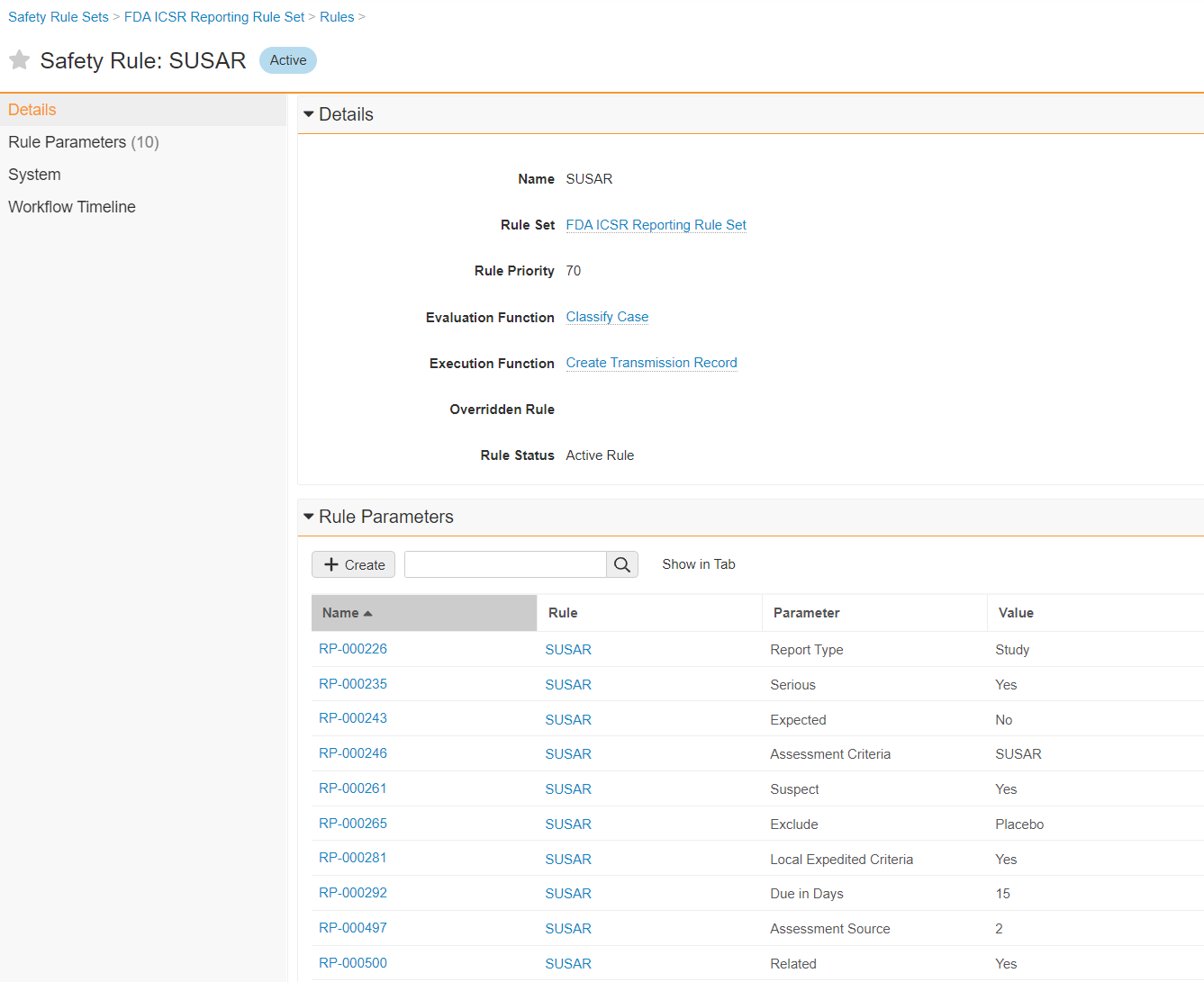
The following sections describe the parameters the system supports for Safety Rules.
For relevant parameters, the table identifies how the system evaluates parameters according to the Product Selection setting on the rule set. See Configure Reporting Rules Product Selection for more information.
Note The system uses one method to evaluate each rule set parameter. If an admin set the Product Selection to use the most conservative evaluation, the system uses this method for parameters identified below.
Reporting Rule Parameters
The following table describes the reporting rule parameters that the system evaluates. The Type column identifies whether a parameter is an input or output parameter. Input parameters evaluate Case criteria to find a matching rule. Output parameters control how the Transmission is generated.
| Parameter | Type (Input/Output) | Description | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Report Type | Input |
The Case's Report Type ( The value must be a Report Type that is configured in the Controlled Vocabulary. The system evaluates this parameter for Report Types with the same E2B code. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study Type | Input |
The Case's Study Type ( The EMA rule set uses the Study Type parameter to differentiate between clinical trials and other study types. The value must be a Study Type that is configured in the Controlled Vocabulary. The system evaluates this parameter for Study Types with the same E2B code. Note If the Study Type value on the Case is left blank, this parameter is regarded as a Clinical Trial. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Serious | Input | Whether the case meets seriousness criteria. The system evaluates this parameter using the method specified on the rule set:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Life Threatening | Input | Whether the case meets life-threatening seriousness criteria. The system evaluates this parameter using the method specified on the rule set:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fatal | Input | Whether the case meets fatal seriousness criteria. The system evaluates this parameter using the method specified on the rule set:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Expected | Input | Note The following text describes system behavior during general reporting. For information on cross reporting, see Cross Reporting Evaluation of Expectedness Rule Parameter. When evaluating reporting obligations for Global Cases, the system locates the relevant Case Assessment Expectedness records based on the evaluation method specified on the rule set:
The system uses logic to evaluate the appropriate Expectedness records for this parameter. To see the detailed logic, expand the section below:
When evaluating reporting obligations for Localized Cases, the system first considers the Localized Case Assessment Expectedness. If the Expectedness is blank, the Global Case logic described above is applied. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Suspect | Input | The Drug Role (
Note To use the “Suspect or Drug Not Administered” setting, your Admin must enable Extend Definition of Suspect to Drug Not Administered. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AE in Jurisdiction | Input | Evaluates whether the adverse event occurred in the agency's jurisdiction. The system evaluates this parameter using the method specified on the rule set:
You can view the countries in an agency's jurisdiction by going to the Agency-type Organization record in the Business Admin area. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclude | Input |
Evaluates whether the system excludes placebos when evaluating suspect Case Products for a Study Case. This parameter accepts "Placebo" as an acceptable value. Note Submission rules to not apply to Study Products with a Study Product Role of Placebo. Once unblinding is completed, if all Case Products are placebos, Submissions are not generated. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Assessment Criteria | Input | Evaluates whether the Case meets SUSAR or SAE criteria. The system evaluates this parameter using the method specified on the rule set:
This parameter accepts "SUSAR" or "SAE" as acceptable values. Vault Safety automatically assigns case and assessment tags. See How Case SAE and SUSAR Tags are Assigned for more information. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Assessment Source | Input | Evaluates the Case Assessment Source in relation to the Related rule parameter, to consider the source of a causality assessment. This parameter is evaluated as "True" when both Source Type matches this parameter and the Related parameter is evaluated as "Related". The system evaluates this parameter using the method specified on the rule set:
The system evaluates this parameter using the Controlled Vocabulary E2B Code corresponding to the Source Type ( |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Device Report Type | Input |
The Case Device Report Type ( This parameter accepts "Public Health Risk" or "Malfunction Only" as acceptable values. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Downgrade | Input and Output |
Evaluates whether the current Case’s seriousness, expectedness, and relatedness are downgraded from the previous Case version. The current Case’s Most Conservative Product/Assessment (MCP/MCA) is compared against the previous Case version based on the following Seriousness/Expectedness/Relatedness priority list:
Expand the following drop-down section to see the Most Conservative Product/Assessment (MCP/MCA) criteria that Vault Safety uses for this parameter.
The system determines whether the current Case’s seriousness, expectedness, and relatedness are downgraded from the previous Case version depending on the Downgrade parameter value, as shown in the table below.
This parameter also controls whether the Downgraded flag on the Transmission is turned on. Note This parameter is not supported for Localized Case Assessment Due Date calculation. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transmission Reason | Input and Output | This parameter is evaluated using the previous Transmissions to the same reporting destination and sets the Reason (
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Registration Type | Input | The Registration Type ( The value must be a registration type that is configured in the Controlled Vocabulary. This parameter is used in PMDA reporting rules only. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rule Execution Level | Input | The Rule Execution Level (
If set to Global Case, the rule set is evaluated when the Evaluate Reporting Obligations action is run on the Global Case only. If set to Localized Case, the rule set is evaluated when the Evaluate Reporting Obligations action is run on the Localized Case only. If left blank, the rule set is evaluated when the Evaluate Reporting Obligations action is run on either the Global or Localized Case. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PMDA Reporting Category | Input |
The PMDA Reporting Category ( This parameter accepts a comma separated-list of the active values from within the PMDA Reporting Category picklist. This parameter applies only when using PMDA ICSR Reporting Rule Set Version 1.0. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infection | Input | Evaluates whether the Localized Case includes a Special Adverse Event ( This parameter is used in PMDA reporting rules and is evaluated only when the rule is run on Localized Cases. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special Report Classification | Input | The Special Report Classification ( This parameter is used in PMDA reporting rules. Special Report Classification is an optional field. If this parameter is set to “No”, the rule is evaluated when the Special Report Classification field is blank. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previously Localized | Input | If this parameter is set to “Yes”, the rule evaluates whether a previous version of the Case has a Completed/ACK Accepted Submission to the same agency where the One Last Time (OLT) rule is not evaluated as “True”. If this parameter is set to “All Transmission States”, the rule evaluates if a previous version of the Case has a Submission in any state (except Inactive or a Deleted state type) to the same agency where OLT is not evaluated as “True”. Note The system cannot automatically set OLT to “True” on Local Reporting Detail-based Localized Submission records. This is a known limitation that will be addressed in a future release. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Related | Input | Evaluates whether a causality assessment categorized the adverse event as related to the suspect product. The system evaluates this parameter using the method specified on the rule set:
This parameter is evaluated as "Related" when the relevant Case Assessment contains at least one Case Assessment Result with the Causality Established ( |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previously Submitted | Input | Evaluates whether previous Case versions have been submitted. The logic used by the system is based on whether the parameter is set to "Yes" or "All Transmission States" as follows:
Note This parameter is not supported for Localized Case Assessment Due Date calculation. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Upgrade | Input |
Evaluates whether the current Case’s seriousness, expectedness, and relatedness are upgraded from the previous Case version. The current Case’s Most Conservative Product/Assessment (MCP/MCA) is compared against the previous Case version based on the following Seriousness/Expectedness/Relatedness priority list:
Expand the following drop-down section to see the Most Conservative Product/Assessment (MCP/MCA) criteria that Vault Safety uses for this parameter.
The system determines whether the current Case’s seriousness, expectedness, and relatedness are upgraded from the previous Case version depending on the Upgrade parameter value, as shown in the table below.
Note This parameter is not supported for Localized Case Assessment Due Date calculation. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclude MedDRA Query | Input |
Evaluates if all Case Adverse Events match with terms defined within a MedDRA Query (SMQ/CMQ). If all Case Adverse Events match, a Transmission is not generated.
Any value entered for this parameter must correspond to an active |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Include MedDRA Query | Input |
Evaluates whether at least one Case Adverse Event matches a term defined within a MedDRA Query (SMQ/CMQ). If there is a match, a Transmission is generated.
Any value entered for this parameter must correspond to an active |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reporting Scenario | N/A | Evaluates the reporting rule for potential cross reporting scenarios, if listed in this parameter. If this parameter is left blank, the system evaluates the Case for general reporting only. To use cross reporting, specify one or more cross reporting scenarios. Specify “General Reporting” along with cross reporting scenarios if the rule should general reporting too. This parameter accepts the following options:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Product | Input | Evaluates whether a Case contains a specific Product. This parameter accepts a comma-separated list of active Product ( General Reporting: This parameter evaluates as "True" when a Case contains a Case Product with a matching API Name and a Drug Role of Suspect, Interacting, or Drug Not Administered. Cross Reporting: If Substitute Product/Study for Cross Reporting1 is enabled, then for X→M cross reporting scenarios, this parameter evaluates as "True" when the provided Product’s Registration is evaluated for cross reporting. If Substitute Product/Study for Cross Reporting is not enabled, this parameter is evaluated as for General Reporting above. Note To include Case Products with a Drug Role of Drug Not Administered in this parameter, your Admin must enable Extend Definition of Suspect to Drug Not Administered. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study | Input | Evaluates whether a Case is associated with a specific Study.
This parameter accepts a comma-separated list of active Study API Names. General Reporting: This parameter evaluates as "True" when the Study ( Cross Reporting: If Substitute Product/Study for Cross Reporting1 is enabled, then for X→I cross reporting scenarios, this parameter evaluates as "True" when the provided Clinical Trial Study’s Registration is evaluated for cross reporting. If Substitute Product/Study for Cross Reporting is not enabled, this parameter is evaluated as for General Reporting above. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiable Patient Definition | Input | Evaluates whether the Case has an identifiable patient. The following list describes how the system evaluates this parameter, depending on the value specified for a reporting rule set:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Local Expedited Criteria | Output | Controls the Local Expedited Criteria field on the Transmission. This parameter accepts the following values:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Due in Days | Output | The Transmission due date, in days. The earliest Transmission due date is also populated in the Case Due Date field. For PMDA transmissions, system behavior depends on your Admin’s configuration of the Japan Localization record. See the following considerations:
This parameter accepts a positive whole number value. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Due in Days Override | Output | For inherited rules, you can override the due date from the parent rule. For example, if a parent rule calculates Due in Days as 15 days, enter "7" in this field to override the Due in Days value to seven (7) days. This parameter accepts a positive whole number value. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Due in Days Adjustment | Output | For inherited rules, you can adjust the due date from the parent rule. For example, if a parent rule calculates Due in Days as 15 days, enter "-3" in this field to override the Due in Days value to 12 days. This parameter accepts a positive or negative whole number value. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mask PII | Output | Evaluates whether the Case requires Personal Identifiable Information (PII) masking for Submissions. The following list describes how the system evaluates this parameter, depending on the value specified for a reporting rule:
Note This parameter only applies to Submissions. Distributions snapshot masking options from the Reporting Family. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exceptions to PII Masking | Output | Evaluates whether the Case requires exceptions to PII masking for Submissions. This parameter is only evaluated if the Mask PII parameter is in use. This parameter accepts a comma-separated list of any of the following picklist values: blank_fields__v, parent_sex__v, patient_sex__v If a reporting rule specifies this parameter, the Exceptions to Patient Content Protection ( Note This parameter only applies to Submissions. Distributions snapshot masking options from the Reporting Family. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transmission Profile Override | Output |
If a reporting rule specifies this parameter and the rule executes resulting in a Submission/Distribution, the Transmission record will have the Transmission Profile populated from the value in the rule parameter. This value will override any defaults selected on Product/Study Registrations or any defaulting logic based on the type of the products selected in the Case.
The parameter accepts the API Name of the appropriate Transmission Profile.
The value entered must correspond to an active |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Suppress File Generation | Input | If this parameter is set to “Yes”, when a Transmission record is created that uses this rule, initial file generation is suppressed. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Product Registration Type | Input |
Evaluates whether a Case contains a Product of the specified Product Registration Type.
This parameter accepts a comma-separated list of active Product Type ( The acceptable Product Types include the following:
If your Vault contains custom Product Types, these can be added to the Product Registration Type parameter if required. The rule will pass if any Product on the Case has a Product Registration whose Registered As field matches one of the specified Product Registration Types within the jurisdiction of the agency destination being evaluated. If this field is left blank, the rule will pass if the Case contains a Product with one of the following Product Types:
In addition, for Cross Reporting (X→M Scenarios), the parameter will evaluate the Transmission Product Type of the registration which is generating a cross reporting obligation.
Note For Cross Reporting (X→I Scenarios), the Product Registration Type parameter is not supported and so will always pass. When evaluating a Distribution, Vault Safety obtains the Transmission Product Type as follows:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. The Substitute Product/Study for Cross Reporting setting is located under Admin > Settings > ICSR Settings. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
