Learn how to update your Vault’s configuration to enable the adverse event watchlists feature.
About the Feature
Adverse event watchlists were added in Veeva Safety 19R3.2 Limited Release, and 20R1 General Release. Upgraded Vaults must perform the following configuration to enable this feature.
An additional watchlist feature was added to Vault Safety in 22R1 to allow Admins to configure seriousness criteria on a watchlist and configure watchlists independent of Products or Studies. You must perform these configurations to enable this feature.
Once you have enabled this feature, Configure Adverse Event Watchlists provides more information about how to use it.
Configuration Checks for All Vaults
Go to Configuration > Objects > Watchlist. From the Layouts tab, open the Watchlist Detail Page Layout.
Ensure the Watchlist Detail Page Layout matches the settings and order described in the following table that lists the fields or columns each section should have. Add/remove fields or columns to match the list.
| Section | Settings | Fields/Columns |
|---|---|---|
| Details |
|
|
| Updates to Case Fields |
|
|
| MedDRA Terms |
|
|
| Workflow Timeline | Default Settings | |
| System Information |
|
|
(20R1) Enable Adverse Event Watchlists
You must update the fields, layout, and layout rules for the Watchlist object.
Update the Watchlist Object
To edit the Watchlist object, in the Admin area, go to Configuration > Objects > Watchlist.
Fields
On the Watchlist > Fields tab, update the following fields:
| Field | Changes |
|---|---|
| Expedited | Select Display in default lists and hovercards. |
| MedDRA Version | Select Display in default lists and hovercards. |
| Organization | Select Display in default lists and hovercards. |
| Product |
|
| Study |
|
| Study Product | Select Display in default lists and hovercards. |
| Watchlist Tag | Select Display in default lists and hovercards. |
List Layout
On the Watchlist > List Layout tab, edit the List Layout to match the following order:
- Organization
- Product
- Study
- Study Product
- Expedited
- Watchlist Tag
- MedDRA Version
Detail Page Layout Rules
Go to Watchlist > Layouts > Watchlist Detail Page Layout > Layout Rules, and then add the following layout rules:
| Rule Label | Hide the following Layout Items | IF this Layout Rule Expression is TRUE |
|---|---|---|
| Product Selected |
|
not(isBlank(product__vr.name__v)) |
| Study Selected |
|
not(isBlank(study__vr.name__v)) |
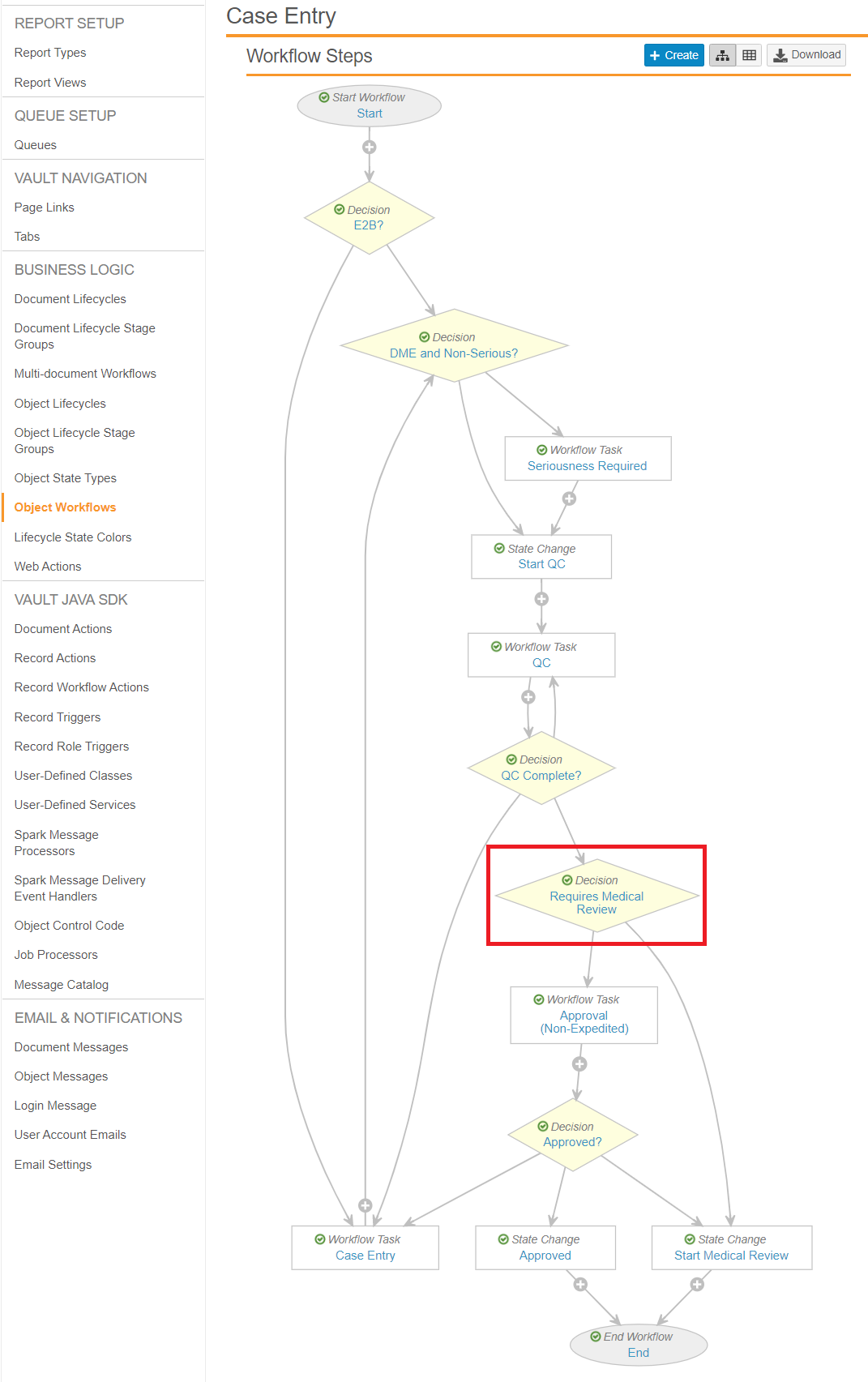
Update the Case Entry Workflow
Go to Object Workflows > Case Entry, and then update the Requires Medical Review workflow step to match the following settings:
- Type: Decision
- Next Steps: Determined by the rules below
- Rule 1: If Expedited = Yes then Start Medical Review
- Else Rule 2: If Watchlist Tags includes AESI then Start Medical Review
- Else: Approval (Non-Expedited)
(22R1) Enable Always Serious and Product or Study-Independent Watchlists
You must update the layout and validation rules for the Watchlist object.
Update the Validation Rules for the Watchlist Object
To edit the Watchlist object, in the Admin area, go to Configuration > Objects > Watchlist.
- Navigate to Admin > Configuration > Objects > Watchlist Validation Rules > Product or Study is Mandatory.
- Update the Validation Expression field to the following expression:
(not(isBlank(product__vr.name__v)) && (isBlank(study__vr.name__v)) ||
((isBlank(product__vr.name__v))) && not(isBlank(study__vr.name__v)) ||
((isBlank(product__vr.name__v))) && (isBlank(study__vr.name__v)))