You can bulk unblind all Cases under a Study using the bulk unblind operation.
About Bulk Unblind
Once your blinded clinical trial ends, you can use the bulk unblind operation to unblind all Cases under a Vault Safety Study as part of the end of study reconciliation. Bulk unblind removes blind protection for previously unblinded Cases and maps product information for blinded, closed Cases. Cases currently being processed are not modified by bulk unblind.
You can initiate bulk unblind from a Study record. You cannot use bulk unblind for Cases across different Studies. For instructions to unblind an individual Case for SUSAR reporting, see Manage Case Blinding.
If your Admin has configured your Vault to isolate blinded product information, see Bulk Unblind Isolated Blinded Product Information.
Procedure Overview
At a high-level, bulk unblind involves the following steps:
- Prepare a CSV file with the study participants
- Trigger bulk actions from the Study
- Complete the Bulk Unblinding Screen
Audit Logging
When the bulk unblind operation runs, all changes are recorded in the applicable audit log as System on behalf of {user}.
Prerequisites
- You must be a Business Administrator with access to the target Study to perform bulk unblind.
- The Study must be configured with Study Arms.
- Ensure your Vault has the necessary configuration to enable this feature.
Prepare the Study Participants CSV File
You must upload a CSV file to complete the Bulk Unblinding screen. The CSV file should list each patient in the study and the treatment group they were in.
See the following considerations when preparing the CSV file:
- The file can contain up to 20,000 rows.
- Each row must be unique.
- A Case’s Investigational MRN or Patient ID must match a row to be included in the unblinding operation. If a Case doesn’t match a patient, it is still included in the summary report.
- Each Case can map to exactly one row.
- Each row can map to any number of Cases, or none.
CSV Sample
See the following example CSV list:
patient_initials,investigational_mrn,study,study_arm,randomization_number
AR,pt001,STU-004,Investigational,jfgoubas7823nf982
MM,pt002,STU-004,Investigational,fdggf3212fsdf
SK,pt003,STU-004,Placebo,f32c6665ggdg
FL,pt004,STU-004,Active Comparator,324dsgasd235
EW,pt005,STU-004,Active Comparator,fhhybg57674
SKI,pt006,STU-004,Placebo,mnut564
AL,pt007,STU-004,Investigational,khuyjh754d43
RP,pt008,STU-004,Investigational,75hgf65jhgg
SS,pt009,STU-004,Placebo,hrfg5453
CSV Format
The following table describes the columns supported in the CSV file:
| Column | Character Limit | Description |
|---|---|---|
patient_initials |
60 | Each row must specify at least one patient identifier to match to a Case, which can be This value matches to the Vault Safety Case > Patient Initials field. |
investigational_mrn |
20 | Each row must specify at least one patient identifier to match to a Case, which can be If both values are specified and there is a conflict on a matching Case, the This value matches to the Vault Safety Case > MRN - Investigation field. |
study |
- | Identifies the study to match Cases for the bulk unblind process. The value must exactly match either the Name on the Study linked to the Case, or the Study Number on the Case. This value matches to either of the following Vault Safety fields:
|
study_arm |
- | Identifies the treatment group the patient was assigned. The value must exactly match a valid Study Arm name. This value matches to the Vault Safety Case > Study Arm > Name field. |
randomization_number |
200 | (Optional) Identifies the patient's randomization number from the clinical trial randomization system. Vault maps this value to the Randomization Number field to the follow-up Case version. |
Trigger the Bulk Unblind User Action
Initiate bulk unblinding using the Launch Bulk Actions user action from the All Actions menu on the Study.
Once you trigger this action, the Bulk Unblinding screen appears.
Complete the Bulk Unblinding Screen
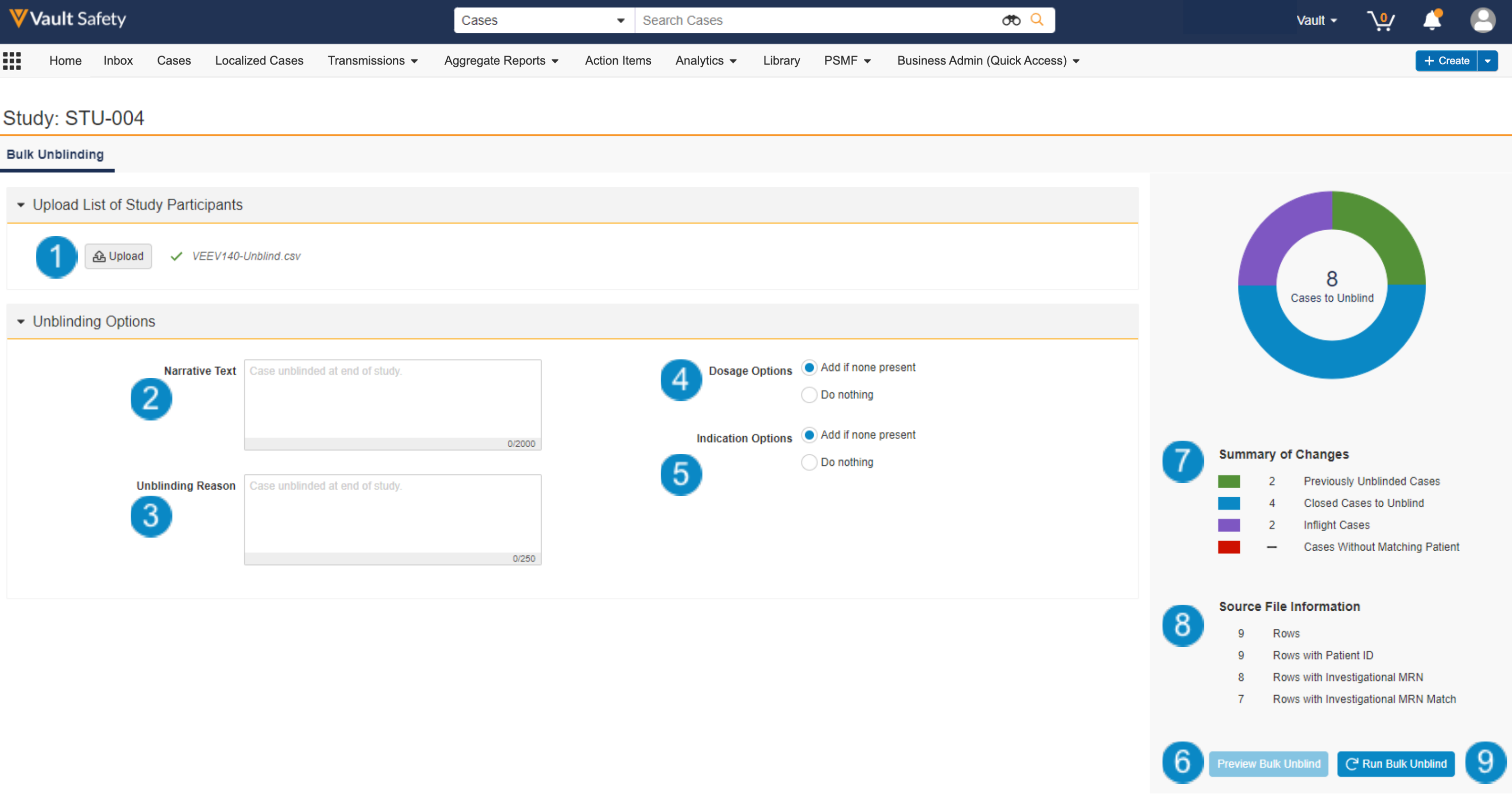
The following image shows the Bulk Unblinding screen:
- Upload List of Study Participants: Upload the CSV file that lists each patient in the study and the treatment group they were on.
- Narrative Text: You can enter up to 2,000 characters, which will be appended to the narrative document on follow-up Cases created as a result of the bulk unblind action.
- Unblinding Reason: You can enter up to 250 characters, which will be populated in the Unblinded Reason field on follow-up Cases created as a result of the bulk unblind action.
- Dosage Options: To map Dosages to follow-up Cases created as a result of the bulk unblind action, select “Add if none present”.
- Indication Options: To map Indications to follow-up Cases created as a result of the bulk unblind action, select “Add if none present”.
- Preview Bulk Unblind: Select this button to process the uploaded CSV file. Once you select this button, Vault validates the contents of the CSV file and shows a summary of the Cases found for the bulk unblind operation.
- Summary of Changes: Once Vault has processed the CSV file, this section shows a summary of the Cases found. See Summary of Changes: Categories & Colors for more details.
- Source File Information: Once Vault has processed the CSV file, this section displays a summary of the file contents.
- Run Bulk Unblind: Once you have confirmed the settings and summary are correct, select this button to run the bulk unblind action.
Note: You can navigate to other pages while the operation runs in the background. Vault sends a notification once the bulk unblind operation is complete.
Summary of Changes: Categories & Colors
Cases are divided into colors by category. The following table outlines different categories and the bulk unblind impact on each:
| Category | Color | Description |
|---|---|---|
| Previously Unblinded Cases | Green | These Cases have been unblinded, which is identified by the following attributes:
These Cases can be in any lifecycle state, including a Closed state. Once the bulk unblind action runs, Vault removes blind protection from these Cases and all related documents. |
| Closed Cases | Blue | These Cases meet the following criteria:
Once the bulk unblind action runs, Vault creates a new Case version with follow-up information added. |
| Inflight Cases | Purple | These Cases meet the following criteria:
Vault does not make any changes to these Cases through the bulk unblind action. |
| Cases Without a Matching Patient | Red | These Cases are associated with the Study but did not match a patient identifier in the CSV file. Vault does not make any changes to these Cases through the bulk unblind action. |
Summary Report
Vault Safety generates a summary report for each successful bulk unblind operation. Once the operation is complete, the Vault notification includes a link to the summary report. Vault attaches the Summary Report to the Study.
The summary report is an XLSX file that includes the following tabs:
| Tab | Description |
|---|---|
| Input | A summary of the input options specified on the Bulk Unblinding screen, the user who ran the action, and the date and time when the action ran. |
| Summary | A summary of the number of Cases in each category. |
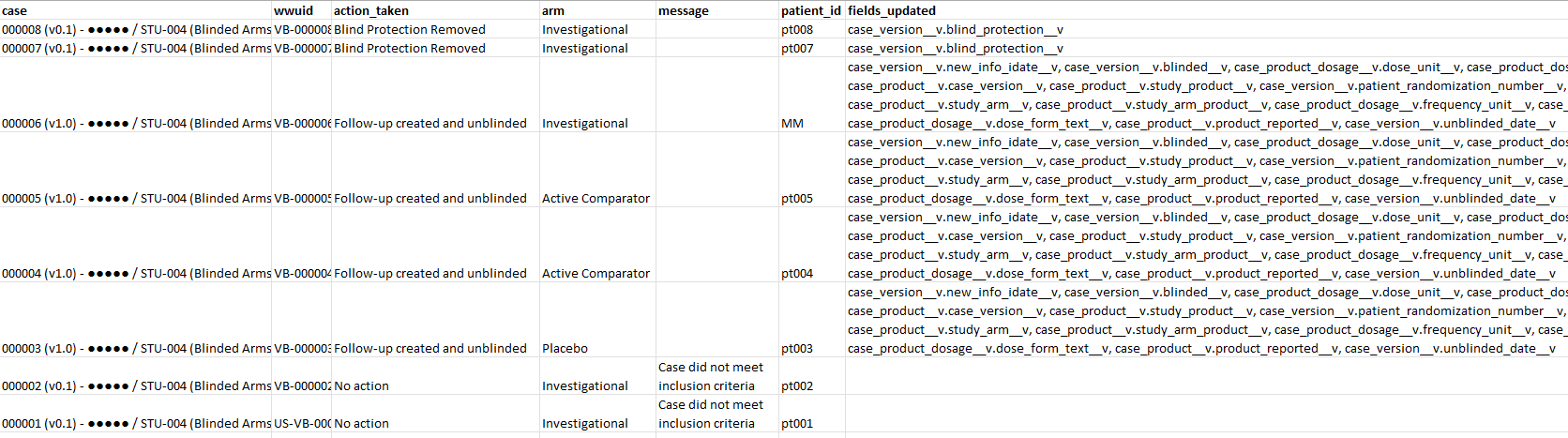
| Activity Log | A detailed log of each Case evaluated by the bulk unblind action and the action taken. The Activity Log includes the following columns:
|
Bulk Unblind Summary Report Activity Log:
Bulk Unblind Study Modifications
Once the bulk unblind operation runs, Vault makes the following changes to the source Study:
- Sets the Study Blinded field to “No”
- Sets the Study lifecycle state to End of Study Reconciliation
- Adds the bulk unblind Summary Report as an attachment to the Study
Bulk Unblind Follow-Up Case Updates
When the bulk unblind action runs, Vault creates a follow-up version of all closed Cases that have not been unblinded (blue category). The follow-up Case is assigned the End of Study Unblinding lifecycle state.
New data is not added to the previous Case version, and the previous version moves to the Superseded lifecycle state.
For follow-up Cases created as a result of the bulk unblind action, Vault updates certain information on the new Case version. Some changes are conditional, which are identified in the following table:
| Case Property | Changes |
|---|---|
| Narrative Document | Vault appends any text entered in the Narrative Text field on the Bulk Unblinding screen to the narrative document. |
| Randomization Number Field | When the Randomization Number field is empty, Vault maps the Randomization Number if it is available in the CSV file. The value is truncated at 60 characters to adhere to the field limit. |
| New Info Date Field | Vault updates the New Info Date field to the point in time when the operation was run in the initiating user's timezone. |
| Unblinded Reason Field | Vault populates the Unblinded Reason field with the text entered in the Unblinding Reason field on the Bulk Unblinding screen. |
| Unblinded Date Field | Vault updates the Unblinded Date field to the point in time when the operation was run in the initiating user's timezone. |
| Unblinded By Field | Vault sets the Unblinded By field to Sponsor. |
| Study Type Field | If the Study Type is blank, Vault sets the field value to the Study Type specified on the Study. |
| Blinded Field | Vault sets the Blinded field to No. |
| Case Product Record | Vault updates the Case Product for the blinded Study Product with the actual Study Arm Product the patient was administered in the treatment group. |
| Case Product Dosage Record | When the Dosage Options field on the Bulk Unblinding screen is set to "Add if none present", Vault maps the dosage from the associated Study Arm Product. This change only occurs if the Case Product does not contain any Dosage records. |
| Case Product Indication Record | When the Indication Options field on the Bulk Unblinding screen is set to "Add if none present", Vault maps the Indication field value from the associated Study Arm Product. If the Study Arm Product does not have an Indication specified, then Vault maps each Indication under the Study. This change only occurs if the Case Product does not contain any Indication records. |