Learn how Vault generates the CIOMS I form from a Case. For information on how Vault generates a localized CIOMS I form for Localized Cases reportable to China, see CIOMS I (Chinese) Generation Data Mapping.
The following list describes how Vault handles certain data while generating a CIOMS I form:
- For events, diagnoses, indications, test names, and medical conditions, Vault always populates the MedDRA Lowest Level Term (LLT) when there is a coded value available. If there is no MedDRA term available, the reported term is used.
Note: Your Admin can configure your Vault to populate the MedDRA Preferred Term (PT) for adverse events instead of the LLT. See Enable Export Product (Reported) to CIOMS I and FDA 3500A for details on setting the default value for export.
- Dates are populated using the format
dd-mmm-yyyy. Blank dates are populated as dashes (---). - Certain fields may be masked, depending on the blinding settings on the Case or masking settings on the Distribution. Generate Masked Distributions describes the fields that may be masked.
- Open-label Products within blinded Studies may be unmasked, depending on your Admin’s configuration of Show Unmasked Open-Label Products on Blinded Forms
Optional Configuration
To evaluate Case Products with the Drug Role of Drug Not Administered as suspect, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered. Otherwise, Vault evaluates only Case Products with the Suspect or Interacting Drug Role as suspect.
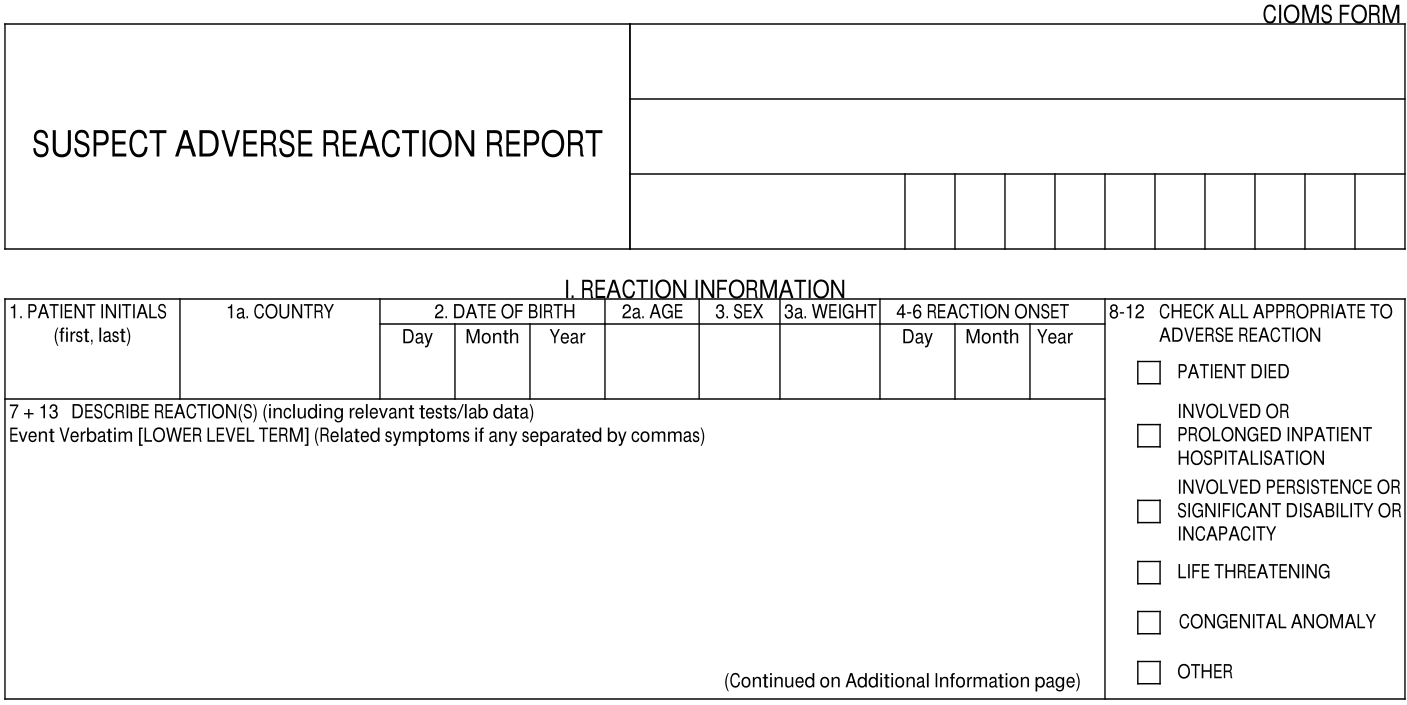
I. Reaction Information
| CIOMS I Field | Populated Value |
|---|---|
| 1 Patient Initials | The value entered in the Patient Initials / ID field on the Case. |
| 1a. Country | The two-letter country code and name of the country is populated based on one (1) of the following fields, ordered by priority:
If none of these fields are populated, no value is exported. |
| 2. Date of Birth | The date entered in the Date of Birth field on the Case. |
| 2a. Age | This value is mapped from one of the following Case fields, ordered by priority:
|
| 3. Sex | The value from the Sex field on the Case. |
| 3a. Weight | This value is mapped from one of the following Case fields, ordered by priority:
|
| 4-6 Reaction Onset | The earliest Onset Date for any Case Adverse Event associated with the Case version. This field is masked if the Onset (reason omitted) field on the Case Adverse Event is "Masked". |
| 7 + 13 Describe Reaction(s) | This field is populated with the following information:
|
| 8-12 Check All | The appropriate checkboxes are selected, based on the Seriousness criteria for each Adverse Event associated with the Case. If there are multiple Seriousness values selected for a Case Adverse Event, Vault populates the checkbox corresponding to the most severe value, according to the following order (from most severe to least):
|
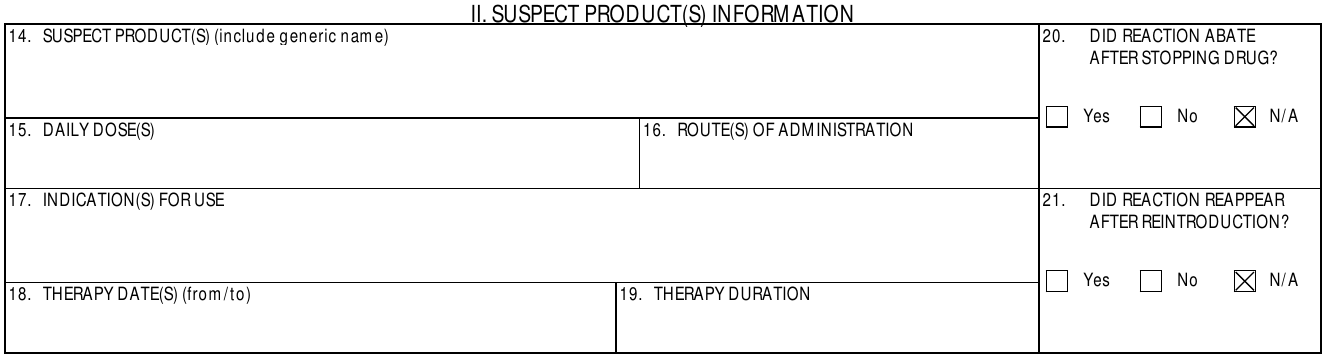
II. Suspect Product(s) Information
| CIOMS I Field | Populated Value | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14. Suspect Product(s) | Vault populates this information for Case Products with an eligible Drug Role:
|
||||||||||||||||||||||||||||||||
| 15. Daily Doses | The values and units in the Dose, Frequency, and Dose Text fields on the primary Case Product Dosage. | ||||||||||||||||||||||||||||||||
| 16. Route(s) of Administration | The value entered in either the Patient RoA or Patient RoA (Reported) field on the associated Case Product Dosage.
For parent-child cases, the routes of administration are listed for both the patient (child) and the parent. |
||||||||||||||||||||||||||||||||
| 17. Indication(s) for Use | The value entered in either the Name (MedDRA) or Indication (Reported) field on the associated Case Product Indication record. | ||||||||||||||||||||||||||||||||
| 18. Therapy Date(s) | This field is populated with the following fields for each associated Case Product Dosage record: [First Admin Date or --- or unk] to [Last Admin Date or --- or unk] |
||||||||||||||||||||||||||||||||
| 19. Therapy Duration | The value and unit entered in the Duration field on the associated Case Product Dosage record. Vault updates the Duration field value to "Ongoing" if any of the following values are in the Action(s) Taken field:
|
||||||||||||||||||||||||||||||||
| 20. Did Reaction Abate After Stopping Drug? | Vault uses the following fields to calculate whether a dechallenge test occurred and populate the appropriate checkbox:
The following table outlines how different values in these fields populate this field on the CIOMS I form:
|
||||||||||||||||||||||||||||||||
| 21. Did Reaction Reappear After Reintroduction? | Vault uses the Reaction Recurrence field on the primary Case Assessment to determine whether a rechallenge test occurred and populate the appropriate checkbox.
|
||||||||||||||||||||||||||||||||
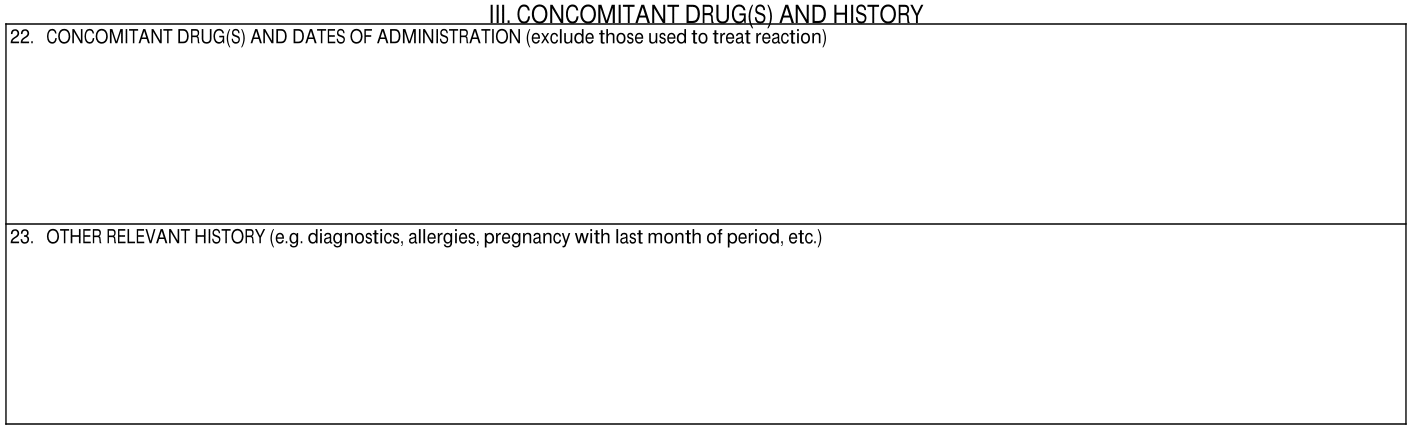
III. Concomitant Drug(s) and History
| CIOMS I Field | Populated Value |
|---|---|
| 22. Concomitant Drug(s) and Dates of Administration | The following information is repeated for each associated Case Product record, populated from the following fields:
|
| 23. Other Relevant History | The following information is populated:
|
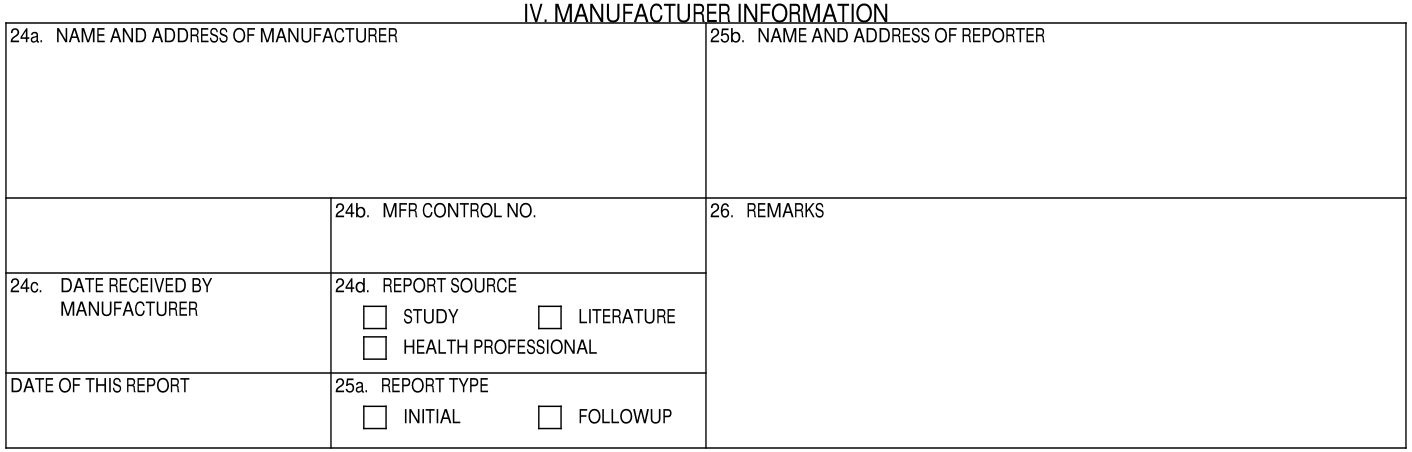
IV. Manufacturer Information
| CIOMS I Field | Populated Value |
|---|---|
| 24a. Name and Address of Manufacturer |
The contact information is populated from the user selected in the Sender User field on the associated ICSR Transmission. When generating a form preview from the Case, the contact information is populated with the contact details for the Case Organization. |
| 24b. MFR Control No. | The value from the UID field on the Case. |
| 24c. Date Received by Manufacturer | Initial Cases: The date is mapped from the Case New Info Date field. If the New Info Date is not populated, the date is mapped from the Case Receipt Date field.
A Case is considered initial when it meets one of the following conditions:
|
| 24d. Report Source | Vault selects Report Source checkboxes as follows:
|
| 25a. Report Type | The appropriate checkbox is selected, depending on whether the Case is initial or follow-up. The Report Type is determined with the following logic:
|
| 25b. Name and Address of Reporter | This field is populated using the name and address on the primary Case Reporter record. |
| Date of This Report | The date when the report was generated, according to the Vault's Timezone. |
| 26. Remarks | The text entered in the CIOMS Remarks field on the Case. |
Footer
The left-side of the CIOMS I footer is timestamped with the date and time when the form was generated, according to the Vault’s Timezone.
The right-side of the footer is populated with the Case UID, appended with the value in the Follow-up Number field on the associated ICSR Transmission.
The follow-up number will be appended when present on the Transmission, even when the Case type is not a Follow-Up Case.
Additional (Overflow) Pages
When Vault generates the CIOMS I form, any text that exceeds field character limits is overflowed to additional pages. The indicator “(…)” and/or “(Continued)” is appended to any field or section that continues on additional pages. Any text on the additional pages is identified with the relevant section and field. Any Case Assessment Results associated with the Case are also listed in the additional pages.
Assessment Results in Overflow Pages
Any Case Assessment Results associated with the Case are listed in the additional pages. For example:
- Cholecap⇄Yellow Fever, Healthcare Professional, EU Method of Assessment, Reasonable Possibility
- *****⇄Yellow Fever, Sponsor, EU Method of Assessment, Reasonable Possibility
Each record is listed in the following format:
For unblinded double-blinded Studies, if there is a specified value in the Assessment Result (Override) field, Vault uses this value instead of the Assessment Result value for unmasked CIOMS I Transmissions and previews. An administrator must perform configurations and contact Veeva Support to request this feature be made available in your Vault.
Note: There is a limitation where if there is a specified value in the Assessment Result (Override) field, Vault will always generate the masked version of aggregate reports. This limitation will be addressed in a future release.
*Product Name Mapping
The way Vault maps the Product name during report generation depends on the Product blinding settings:
- For non-Study Cases, open-label Study Cases, and open-label Products within blinded Studies, the Product name is mapped from the Name (
product_name_v) field on the Case Product record.
Note: Your Admin must configure unmasked export of open-label Products within blinded Studies. If this feature is not turned on, open-label Products within blinded Studies are mapped as described in the next bullet.
- For blinded Study Cases, Vault uses the following logic to map the Product name for blinded Products:
| Criteria (Inputs) | Form Generation | |||
|---|---|---|---|---|
| Does the Study Have Arms? (study_has_arms_v) | Is the Case Blinded? (blinded_v) | Document Masking | Product Name Mapping | Example |
| No | No | Unblinded | Case Product > Name (product_name_v) |
Cholecap |
| No | No | Blinded | ***** | ***** |
| No | Yes or Blank | Unblinded | N/A: Document not generated | |
| No | Yes or Blank | Blinded | ***** | ***** |
| Yes | No | Unblinded | Case Product > Name (product_name_v) |
Cholecap |
| Yes | No | Blinded | Case Product > Blinded Name (Placeholder) (study_product_placeholder__v) |
Cholecap vs. Placebo |
| Yes | Yes or Blank | Unblinded | N/A: Document not generated | |
| Yes | Yes or Blank | Blinded | Case Product > Blinded Name (Placeholder) (study_product_placeholder__v) |
Cholecap vs. Placebo |