Learn how Vault generates the August 2024 version of the FDA MedWatch 3500A form from a Case. For details about the available FDA MedWatch 3500A formats supported by Vault Safety and how Vault handles certain data during form generation, see FDA MedWatch 3500A Generation Data Mapping.
Prerequisite
To enable this version of the FDA MedWatch 3500A form, your Admin must contact Veeva Support and then complete the required configuration. Once configured, Vault generates the August 2024 version of the form, instead of the October 2015 version.
Header
| FDA MedWatch 3500A Field | Populated Value |
|---|---|
| Mfr. Report # | For device Cases, Vault exports the FDA Manufacturer Report Number on the Case Product on the Transmission. If blank, and only one Case Product on the Case related to the Transmission includes an FDA Manufacturer Report Number, Vault exports that value. For non-device Cases and device Cases on which the FDA Manufacturer Report Number is blank, Vault maps the UID on the Case. |
| UF/Importer Report # | Vault does not populate this field. |
| Exemption/Variance # | Vault does not populate this field. |
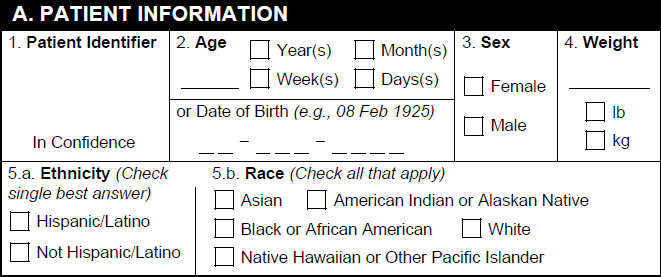
A. Patient Information
| FDA MedWatch 3500A Field | Populated Value |
|---|---|
| 1. Patient Identifier | The value in the Patient Initials / ID field on the Case. If this field is blank, Vault populates the value entered in the Investigation MRN field. |
| 2. Age | The value from one (1) of the following Case fields, ordered by priority:
|
| 2. Date of Birth | The date in the Date of Birth field on the Case. |
| 3a. Sex | The value in the Sex field on the Case as follows:
|
| 3b. Gender | The value in the Gender field on the Case as follows:
|
| 4. Weight | The value from one (1) of the following Case fields, ordered by priority:
|
| 5. Ethnicity | The value from the Ethnicity field on the Case. |
| 6. Race | The value from the Race field on the Case. |
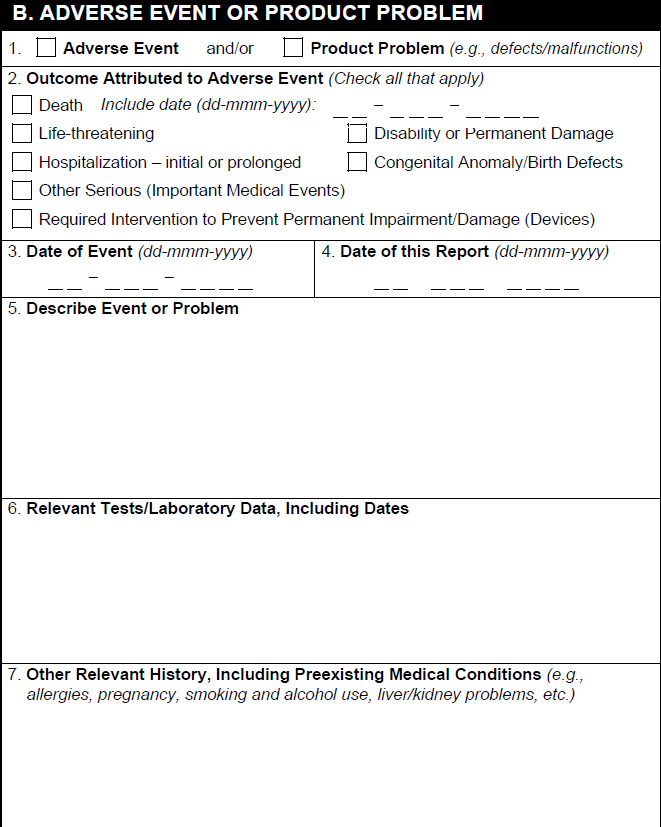
B. Adverse Event or Product Problem
| FDA MedWatch 3500A Field | Populated Value |
|---|---|
| 1. Type of Report | Based on the report type, Vault selects:
|
| 2. Outcome Attributed to Adverse Event | Vault selects the appropriate checkbox based on the Seriousness field in the Details section of the Case. If the adverse event resulted in death, Vault populates the date in the Date of Death field on the Case. |
| 3. Date of Event | The earliest Onset Date for any Case Adverse Event associated with the Case version. |
| 4. Date of this Report | The date when the report was generated, according to the Vault's timezone. |
| 5. Describe Event or Problem | Any text in the Narrative on the Case. After the narrative text, Vault populates any text in the Company Comments field. If the narrative text overflows onto additional pages, Company Comments also appear on the overflow pages. |
| 6. Relevant Test/Laboratory Data | Vault populates a row for each Case Test Result record on the Case, sorted alphabetically and from oldest to latest. Lab Test Results with no Test Date appear at the end of the list. The information exported includes:
Text that exceeds the available space appears on overflow pages. |
| 6. Additional comments | For each Case Test Result record in the B6. Relevant Test/Laboratory Data section, Vault populates the following information when available:
In addition, for adverse events that resulted in death, the following information is populated from each Case Cause of Death record:
Text that exceeds the available space appears on overflow pages. |
| 7. Other Relevant History, Including Preexisting Medical Conditions | The following information is repeated for each Case Medical History record, populated from the following fields:
After listing each medical history line item, Vault populates any text in the Medical History Text field on the Case. |
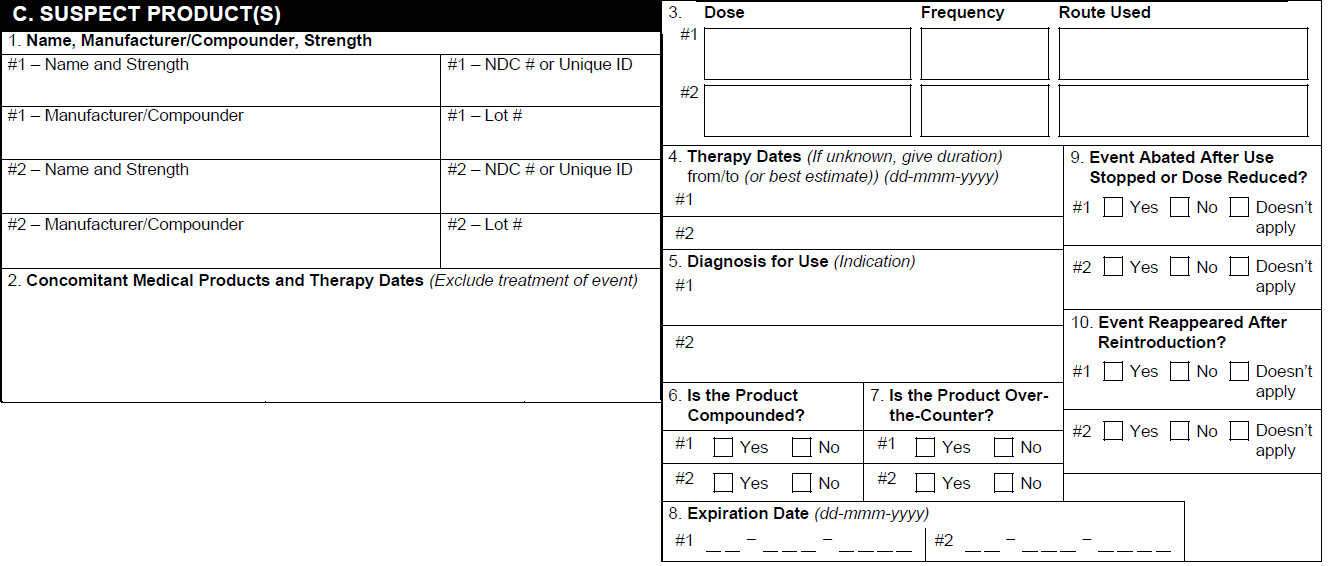
C. Suspect Products
| FDA MedWatch 3500A Field | Populated Value | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Name, Strength, Manufacturer/Compounder | For Case Products assigned a Drug Role of Suspect, Interacting, or Drug Not Administered, Vault populates:
|
||||||||||||||||||||||||||||||||
| 2. List Medical Product and Treatment Given at the Same Time of the Event and Date | For each associated Case Product record, Vault populates:
If the therapy dates are unknown, Vault populates this field with the value from the Duration field. Vault updates the Duration field value to Ongoing if any of the following values are in the Action Taken field:
Note: For Device-only Cases, Vault maps this information to Section D.11 of the form. |
||||||||||||||||||||||||||||||||
| 3. Dose, Frequency, Route Used | For Case Products assigned a Drug Role of Suspect, Interacting, or Drug Not Administered, Vault populates:
|
||||||||||||||||||||||||||||||||
| 4. Treatment Dates/Therapy Dates | Vault populates information from the following Product Dosage fields from the Case: First Admin Date or If the therapy dates are unknown, Vault populates this field ith the value from the Duration field. Vault updates the Duration field value to Ongoing if any of the following values are in the Action Taken field:
|
||||||||||||||||||||||||||||||||
| 5. Diagnosis for use | The value in either the Name (MedDRA) or Indication (Reported) field on the associated Product Indication record on the Case. | ||||||||||||||||||||||||||||||||
| 6. Product Type | Vault maps values from the applicable FDA Product Registration to select checkboxes. | ||||||||||||||||||||||||||||||||
| 7. Expiration Date | The date in the Expiration Date field on the associated Case Product. | ||||||||||||||||||||||||||||||||
| 8. Event Abated after use Stopped or Dose Reduced? | Vault uses the following fields to calculate whether a dechallenge test occurred and populates the appropriate checkbox:
The following table outlines how Vault populates the form based on the field values:
|
||||||||||||||||||||||||||||||||
| 9. Event Reappeared After Reintroduction? | Vault uses the Reaction Recurrence field on the primary Case Assessment to determine whether a rechallenge test occurred and populate the appropriate checkbox.
|
||||||||||||||||||||||||||||||||
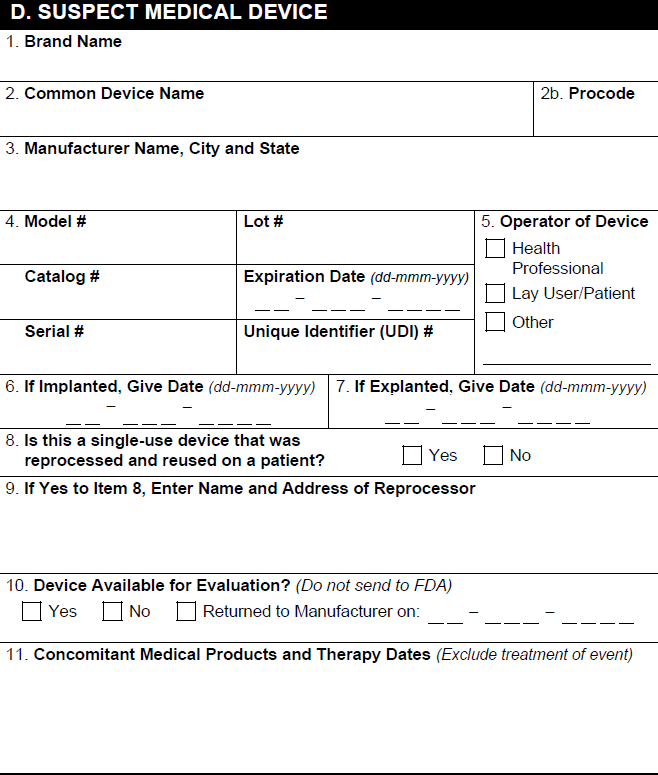
D. Suspect Medical Device
| FDA MedWatch 3500A Field | Populated Value |
|---|---|
| 1. Brand Name | The value in the Product (Reported) field on the Device-type Case Product.
Note: Your Admin can configure your Vault to export the Product (Coded) field by default. See Enable Export Product (Reported) to CIOMS I and FDA 3500A for details. |
| 2a. Common Device Name | The value in the Generic Name field on the Product. |
| 2b. Procode | The value in the Product Code field on the associated Product Registration. |
| 3. Manufacturer Name, City and State | If an organization is selected in the Manufacturer field on the associated Product Family record, Vault populates the associated name, city, and state. Otherwise, Vault populates the value in the Organization field of the associated Product Family. |
| 4. Model # | The value in the Model Number field on the Device-type Case Product. |
| 4. Lot # | The value in the Lot Number field on the Device-type Case Product. |
| 4. Catalog # | The value in the Catalog Number field on the Device-type Case Product. |
| 4. Expiration Date | The value in the Expiration Date field on the Device-type Case Product. |
| 4. Serial # | The value in the Serial Number field on the Device-type Case Product. |
| 4. Unique Device Identifier (UDI)# | The value in the Unique Identifier field on the Device-type Case Product. |
| 5. Operator of Device | The value in the Operator of Device field on the Device-type Case Product. If the value is Other, Vault selects the Other checkbox and populates the value in the Operator of Device (Other) field. |
| 6a. If Implanted, Give Date | The date in the Date Implanted field on the Device-type Case Product. |
| 6b. If Explanted, Give Date | The date in the Date Explanted field on the Device-type Case Product. |
| 7a. Is this a single-use device that was reprocessed and reused on a patient? | Vault selects the appropriate checkbox based on the Reprocessed/Reused field on the Device-type Case Product. |
| 7b. If yes, enter the name and address of the reprocessor | The value in the Reprocessor field on the Device-type Case Product. |
| 8. Was this device ever serviced by a third-party servicer? | Vault selects the appropriate checkbox based on the value in the Third-party servicer for device? field on the Device-type Case Product. |
| 9. Is this Device Available for Evaluation? | Vault selects the appropriate checkbox based on the Device Available field on the Device-type Case Product. If there is a date in the Returned Date field, Vault populates the date and selects the Returned to Manufacturer checkbox. |
| 10. Concomitant Medical Products and Therapy Dates |
For each associated Case Product record, Vault populates values from the following fields:
|
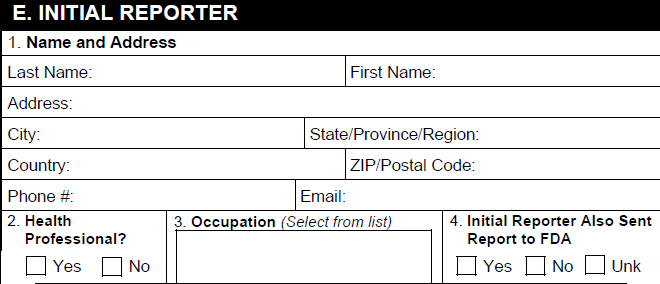
E. Initial Reporter
| FDA MedWatch 3500A Field | Populated Value |
|---|---|
| 1. Name and Address | Vault populates the form fields using the name and address on the primary Reporter-type Case Contact record. |
| 2. Health Professional? | Vault selects the appropriate checkbox based on the value in the Qualification field on the primary Reporter-type Case Contact record. |
| 3. Occupation | The value in the Qualification field on the primary Reporter-type Case Contact record. Vault populates Non-Health Professional if the value is any of the following:
|
| 4. Initial reporter also sent report to FDA | Vault selects the appropriate checkbox based on the value in the Sent to FDA? field on the primary Reporter-type Case Contact record. |
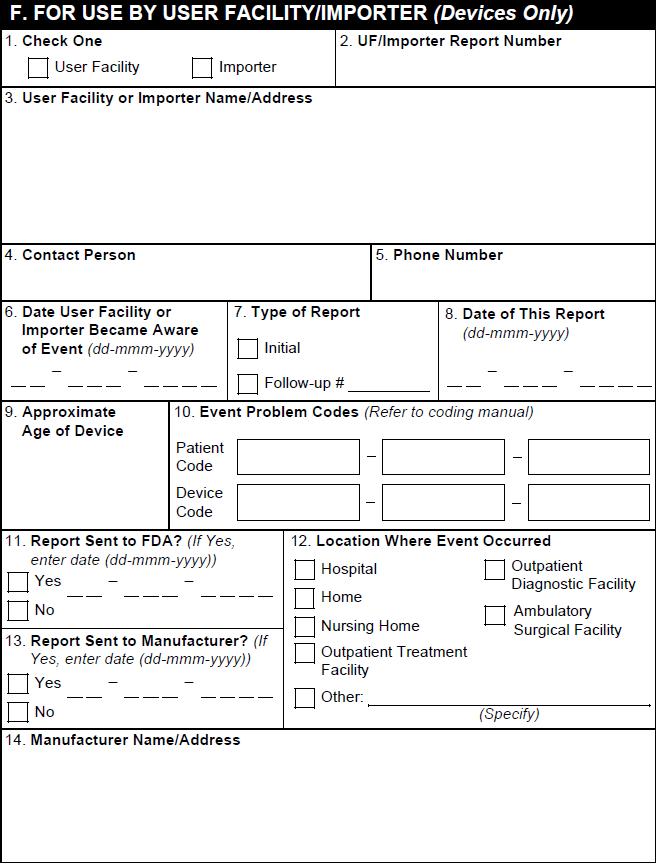
F. For Use by User Facility/Importer (Devices Only)
Note: Vault does not populate section F, but the section is available on the form for manual data entry.
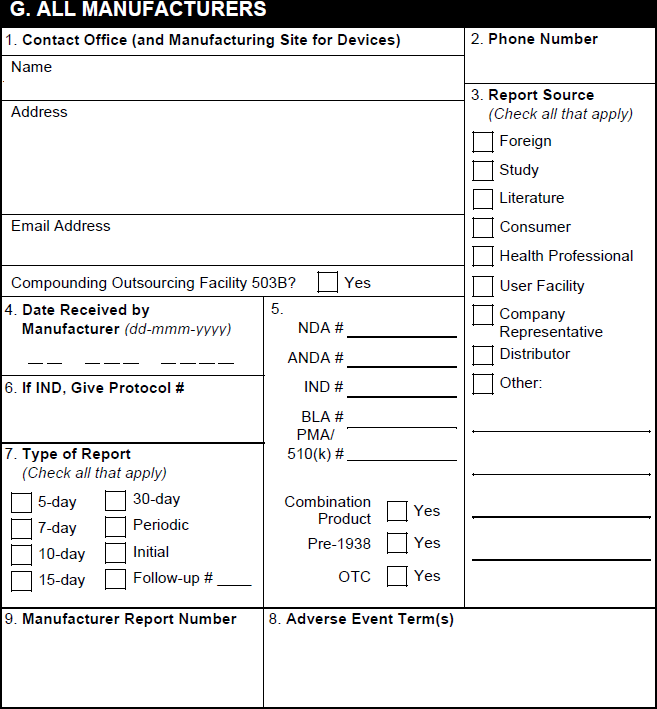
G. All Manufacturers
| FDA MedWatch 3500A Field | Populated Value | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Contact Office (and Manufacturing Site for Devices) or Compounding Outsourcing Facility | Vault populates the contact information for the user selected in the Sender User field on the associated Transmission (Submission or Distribution) record. When generating a form preview from the Case, Vault populates contact details for the Organization on the Case and the phone number for the organization selected in the Manufacturer on the Product record associated with the Case Product. | ||||||||||||||||||||
| 2. Report Source | Vault selects the appropriate checkboxes as follows:
|
||||||||||||||||||||
| 3. Date Received by Manufacturer | Vault uses the following logic to populate this field: Initial Case: Vault maps the date from one (1) of the following Case fields, ordered by priority:
A Case is considered initial when it meets any of the following conditions:
Follow-Up: Vault maps the date from the New Info Date field on the Case, if populated. A Case is considered follow-up when it meets any of the following conditions:
|
||||||||||||||||||||
| 4. NDA # | If the Registration Type field on the associated Product Registration is set to NDA, Vault maps this value from the Registration Number field. | ||||||||||||||||||||
| 4. ANDA # | If the Registration Type field on the associated Product Registration is set to ANDA, Vault maps this value from the Registration Number field. | ||||||||||||||||||||
| 4. IND # | For study Cases with a Study Type of Clinical Trial or blank, if there is a US Study Registration with a Registration Type of IND, Vault populates the IND number from the Study Registration Number field from that Study Registration. In all other scenarios, if the Registration Type field on the associated Case Product Registration is set to IND, Vault takes this value from the Registration Number field. |
||||||||||||||||||||
| 4. BLA # | If the Registration Type field on the associated Product Registration is set to BLA, Vault maps this value from the Registration Number field. | ||||||||||||||||||||
| 4. PMA/510(k) # | If the Registration Type field on the associated Product Registration is set to either PMA or 510k, Vault maps this value from the Registration Number field. | ||||||||||||||||||||
| 4. Combination Product | When any Case Product is part of a Combination Product, Vault selects this checkbox.
Note: For study Cases with Products that are part of a Combination Product, if Study Content Protection is enabled or a blinded regulatory report preview is generated, Vault does not select this checkbox. |
||||||||||||||||||||
| 4. Pre-ANDA | Vault does not support this field | ||||||||||||||||||||
| 4. Pre-1938 | If the International Birthdate on the associated Product record is set to a date earlier than 1938, Vault selects this checkbox. | ||||||||||||||||||||
| 4. OTC | Across all Case Products in sections C and D, if any part of a Product has an FDA registration with the Registered As field set to a Transmission Product Type of OTC Drug or OTC Device, Vault selects this checkbox. | ||||||||||||||||||||
| 4. Compounded Product | Vault selects this checkbox if any Case Product in section C or D includes:
|
||||||||||||||||||||
| 5. If IND/PreANDA, Give Protocol # | The value in the Sponsor Study Number field on the Case. | ||||||||||||||||||||
| 6. Type of Report | Vault selects the appropriate checkbox based on the value in the FDA Report Type field, which is set based on the Due in Days reporting rule parameter. Otherwise, Vault selects checkboxes based on the following logic:
When no report type applies based on the above criteria, Vault selects the Periodic checkbox. Vault populates the Follow-up # value from the Follow-up Number field on the Submission record. |
||||||||||||||||||||
| 7. Adverse Event Term(s) | The value in the Event (MedDRA) field or the Event (Reported) - English field on the primary Case Adverse Event record. | ||||||||||||||||||||
| 8. Manufacturer Report Number | For device Cases, Vault exports the FDA Manufacturer Report Number on the Case Product on the Transmission. If blank, and only one Case Product on the Case related to the Transmission includes an FDA Manufacturer Report Number, Vault exports that value. For non-device Cases and device Cases on which the FDA Manufacturer Report Number is blank, Vault maps the UID on the Case. |
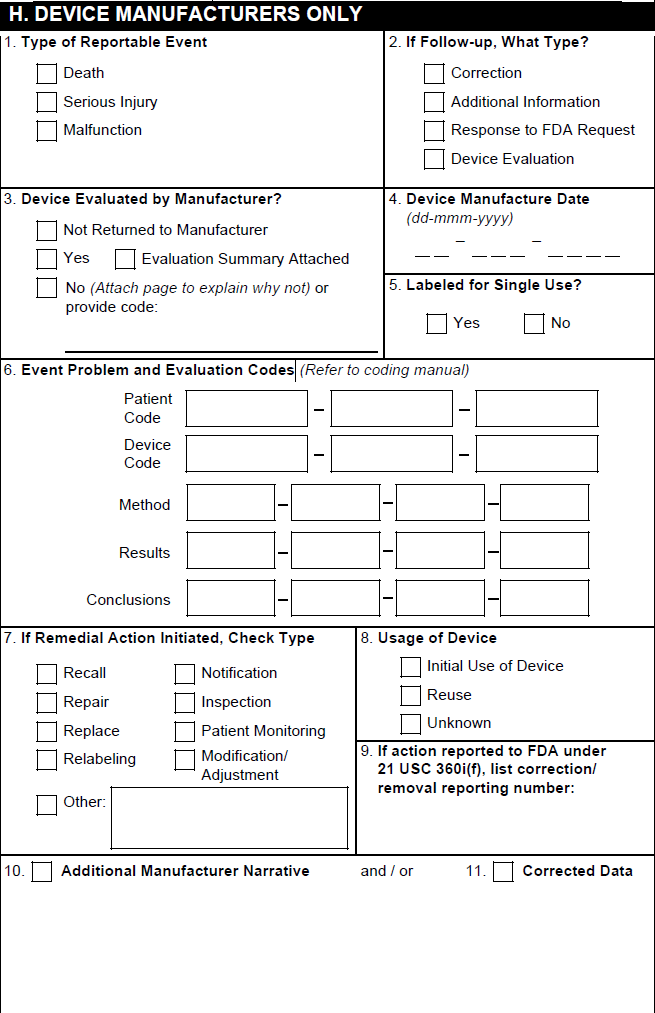
H. Device Manufacturers Only
Note: Section H is populated only for Cases concerning a Combination Product with a Device-type Product Constituent.
| FDA MedWatch 3500A Field | Populated Value |
|---|---|
| 1. Type of Reportable Event | Vault selects the Death checkbox if the Seriousness of any Case Adverse Event contains Results in death. Vault selects the Serious Injury checkbox based on the value in the Device Report Type field on the Case. Vault selects the Malfunction checkbox based on the value in the Malfunction field on the primary Device-type Case Product. If that field is blank, Vault selects a checkbox based on the Device Report Type field on the Case. Vault does not support the Summary Report checkbox or the No. of events summarized field. |
| 2. If Follow-up, What Type? | Vault selects the appropriate checkboxes based on the Device Follow-Up Type field on the Case. |
| 3. Device Evaluated by Manufacturer? | Vault selects the appropriate checkbox based on the Device Evaluated field on the associated Case Product as follows:
|
| 4. Device Manufacture Date | The value from the Manufacture Date field on the associated Case Product. |
| 5. Labeled for Single Use? | For Device-type Case Products, the value from the Single Use field. |
| 6. Adverse Event Problem | Based on the Device Type Code and Device Code on the Device-type Case Product, Vault populates the level 1, 2, or 3 FDA or IMDRF codes. To use IMDRF codes, your Admin must enable the IMDRF Dictionary for Maintenance, Case Processing, and Submissions. Vault populates this section as follows:
|
| 7. If Remedial Action Initiated, Check Type | Vault selects the appropriate boxes using the following priority order:
|
| 8. Usage of Device | Vault selects the appropriate box based on the value in the Device Usage Type field on the associated Device-type Case Product. |
| 9. If action reported to FDA under 21 USC 360i(g), list correction/removal reporting number | The value from the Correction/Removal Reporting Number field on the associated Device-type Case Product. |
| 10. Related Report Number | Vault does not support this field. |
| 11. Additional Manufacturer Narrative | The text in the Additional Manufacturer Narrative field on the associated Device-type Case Product. Text that exceeds the field character limit appears in the overflow pages under the heading H.11 Additional Manufacturer Narrative. |
Note: If your organization is not using Case Product-level device coding, Vault exports FDA codes for one (1) device. This is either the highest-ranked or earliest-entered device on the Case. The code entered on the Case is populated for each of the following:
- Device Problem
- Evaluation Method
- Evaluation Result
- Evaluation Conclusion
Overflow Pages
When generating the FDA MedWatch 3500A form, Vault exports any text that exceeds field character limits to overflow pages. In the applicable field, Vault truncates the text to 15 characters and appends (Continued) to indicate the information is on the overflow pages. Any text on the overflow pages is identified with the relevant section and field names.