Set up an MAH Distribution List to create a reporting obligation between a sponsor and a market authorization holder (MAH).
About MAH Distribution Lists
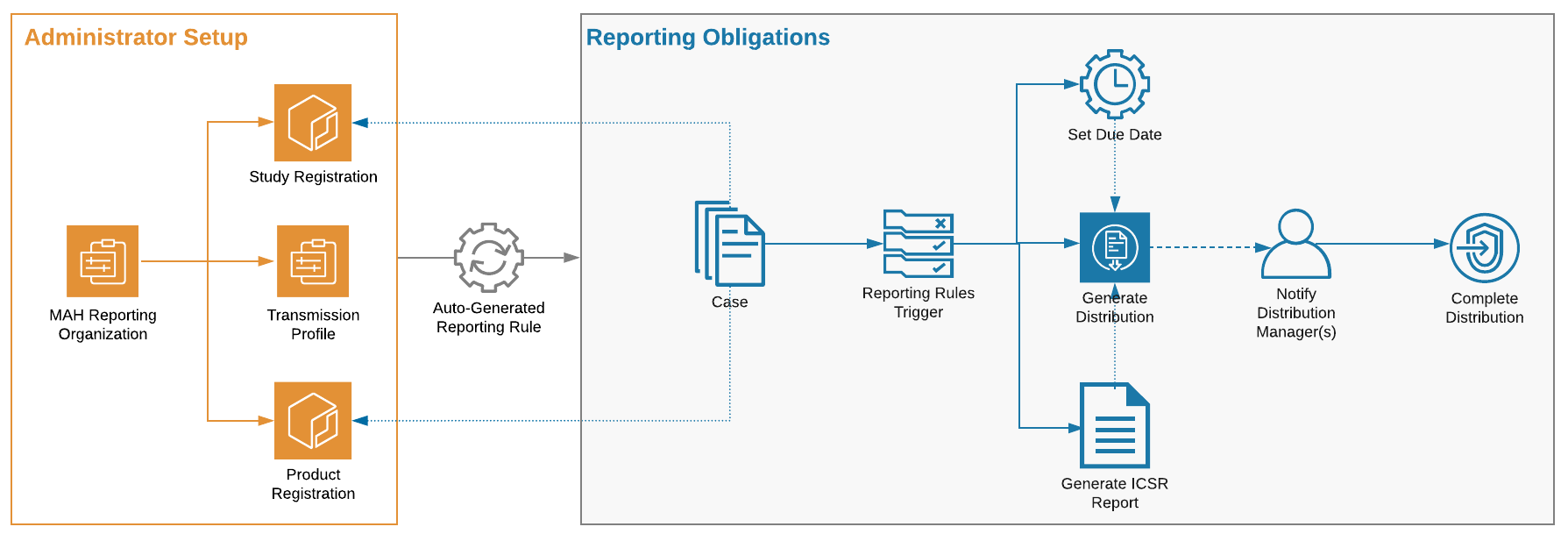
An MAH Distribution List is a type of Reporting Family that admins can configure to automatically generate Distributions to a market authorization holder who handles regulatory reporting on your behalf, in place of generating Submissions to regulatory authorities.
Reporting Obligations are system-managed, meaning users cannot create, edit, or delete these records. When you populate the reporting_organization__v field on a Product Registration or Study Registration, Vault Safety automatically creates an MAH Distribution List.
Note the following considerations for MAH Distribution Lists:
- You can set up one MAH Distribution List per Study or Product Registration.
- By default, Vault Safety assigns the EMA ICSR Reporting Rule Set to all MAH Distribution Lists. You can change the assigned Rule Set after creating the MAH Distribution List.
- Do not set up an MAH Distribution List for a regulatory agency such as FDA or EMA. Reporting obligations through an MAH Distribution List generate Distributions, not Submissions to regulatory agencies.
- In the event where both an MAH Distribution List and one or more Partner Distribution Lists exist for the same Product Registration or Study Registration, Vault Safety creates a Distribution record for each reporting rule, including when both reporting rules are to the same destination.
- The
reporting_organization__vfield may be labelled MAH, Reporting Organization, or a custom name, depending on your Vault’s configuration.
The following figure shows the workflow to set up and use an MAH Distribution List:
Add an MAH Distribution List
To create an MAH Distribution List, assign the reporting_organization__v on a Product Registration or Study Registration.
- Configure an Organization for the market authorization holder.
- To create an MAH Distribution List for a Product Registration, configure a Product Registration and populate the MAH (
reporting_organization__v) field. When a Case contains a Case Product linked with this Product Registration, the MAH reporting obligation will be triggered. - To create an MAH Distribution List for a Study Registration, configure a Study Registration and populate the Reporting Organization (
reporting_organization__v) field. When a Case is associated with a Study linked with this Study Registration, the MAH reporting obligation will be triggered. - Configure the Transmission Profile for the Origin and Destination pair. You can configure an Email Transmission Profile, Manual Transmission Profile, or AS2 Gateway Transmission Profile.
Result
When a Case includes a Product Registration or Study Registration with an MAH Distribution List, that reporting rule applies to the Case.
Note: For Reporting Families set up to use the FDA or EMA reporting ruleset, the system generates Distribution record(s) regardless of the registration country on the Case Product or Study Registration.
View MAH Distribution Lists
You can view system-generated Reporting Obligations on the Business Admin > Objects > Reporting Families page.
Reporting Obligations are named using the default system-generated format, VV-{####}.
Note: If the Reporting Organization value on a Study Registration or the MAH value on a Product Registration changes, any previously-created Distribution records remain active. If necessary, you can deactivate these records manually.
Edit an MAH Distribution List
You can edit specific details of an MAH Distribution List. To edit an MAH Distribution List, navigate to Business Admin > Objects > Reporting Families > [MAH Distribution List].
You can change the name of the distribution list by editing the value in the Name field.
You can change the reporting ruleset on a distribution list from the default EMA ruleset by selecting a ruleset from the Distribution Rules field.
See Standard Reporting Rule Sets for more information on the available Safety Rule Sets.