Learn how Safety’s Reporting Rules Engine simplifies the process of managing Individual Case Safety Report (ICSR) reporting obligations.
About the Reporting Rules Engine
The Reporting Rules Engine comprises of the following parts:
- Safety Rule Sets: Collections of reporting rules (Safety Rules and Safety Rule Parameters) which can be assigned to an Agency, Market Authority Holder (MAH), or Reporting Family.
- Safety Rules: Reporting rules containing one or more Safety Rule Parameters that define the conditions for which a Transmission is generated.
- Transmission Profiles: Generates Transmissions for an origin-and-destination pair with the appropriate ICSR file format.
Admins can set up Safety Rule Sets, Safety Rules, and Transmission Profiles for regulatory agencies, partners, study sites, and market authorization holders such that when the Reporting Rules Engine runs on a Case, Vault creates the necessary Transmissions.
Note: Vault provides Safety Rule Sets, Safety Rules, and Transmission Profiles for certain standard agencies, including the FDA, EMA, MHRA, and PMDA.
The following actions trigger the Reporting Rules Engine to run on a Case:
Depending on your Vault’s configuration, you can trigger these actions manually or Vault can trigger them through the Case lifecycle.
Evaluate Early Notification Obligations
When the Evaluate Early Notification Obligations action runs on a Case, Vault generates Early Notification Reports as required. For more information on Early Notification Reports, see ICSR Transmissions Overview and Create an Early Notification Report.
Evaluate Reporting Obligations
When the Evaluate Reporting Obligation action runs on a Case, Vault generates and assigns due dates for Submissions and Distributions as required. For more information about Submissions and Distributions, see the following articles:
Submission Rules
The Submission Rules field on an Agency assigns a Safety Rule Set to an Agency. While certain standard agencies are preconfigured with a Submission Rule, you can assign Submission Rules to additional Agencies.
To generate a Submission for an Agency, the rules engine must evaluate an active Product Registration or Study Registration for a country within the Agency’s jurisdiction. The Agency field on the associated Country record identifies which regulatory agency has jurisdiction over that country.
Note: If a Product has multiple Product Type registrations based on agency requirements, you can create country-specific Product Registrations.
When evaluating reporting obligations, Vault looks at the country selection on Case Registrations. For general reporting, Vault looks at the following registrations by report type:
- For Cases with a non-Study type of report, Vault evaluates the active Product Registrations.
- For Cases with a Report Type of Study, Vault evaluates the active Study Registrations. However, if the Study Has Unspecified Products field is selected on the associated Study, Vault evaluates the active Product Registrations instead for Cases involving that Study.
Note: After Vault generates Submissions for general reporting, Vault evaluates additional investigational or marketing registrations associated with the Case for the purposes of Cross Reporting.
FDA Registration Details
For automatically generated Submissions to the FDA, Vault populates the appropriate product or study registration details based on existing registrations. Vault does not populate FDA registration details for any Submissions generated through Transmission Output Templates.
Product Registrations
For Submissions generated based on Product Registrations for non-study types of Cases, Vault populates the following registration fields differently for general reporting and cross reporting:
- General Reporting
- Reportable Product: The passing Product of the Case
- Reportable Product Registration: Assigned based on the following priority of marketing Product Registrations for the Reportable Product:
- Earliest Registration Date
- Lowest Registration Number
- Earliest Created Date
- Cross Reporting
- Reportable Product Registration: Assigned based on the following priority of existing FDA marketing Product Registrations:
- Shortest Due in Days
- Earliest Registration Date
- Lowest Registration Number
- Earliest Created Date
- Reportable Product: The Product of the Reportable Product Registration
- Reportable Product Registration: Assigned based on the following priority of existing FDA marketing Product Registrations:
When evaluating Product Registrations for a Case that contains an unknown formulation Product, Vault considers all Product Registrations within the Product Family. When evaluating Registration Numbers of existing Product Registrations to determine the lowest number, Vault ignores any letters in the field values. For example, “C123” is lower than “A321”.
Study Registrations
For Submissions generated based on Study Registrations for study types of Cases, Vault populates the following registration fields for general reporting:
- Reportable Product: The passing Product of the Case
- Reportable Study Registration: Assigned based on the following priority for the Study of the Case:
- Lowest Registration Number
- Earliest Created Date
- Reportable Study: The Study of the Case when the case is a Clinical Trial Study
When evaluating Registration Numbers of existing Study Registrations to determine the lowest number, Vault ignores any letters in the field values. For example, “C123” is lower than “A321”.
General Reporting Example
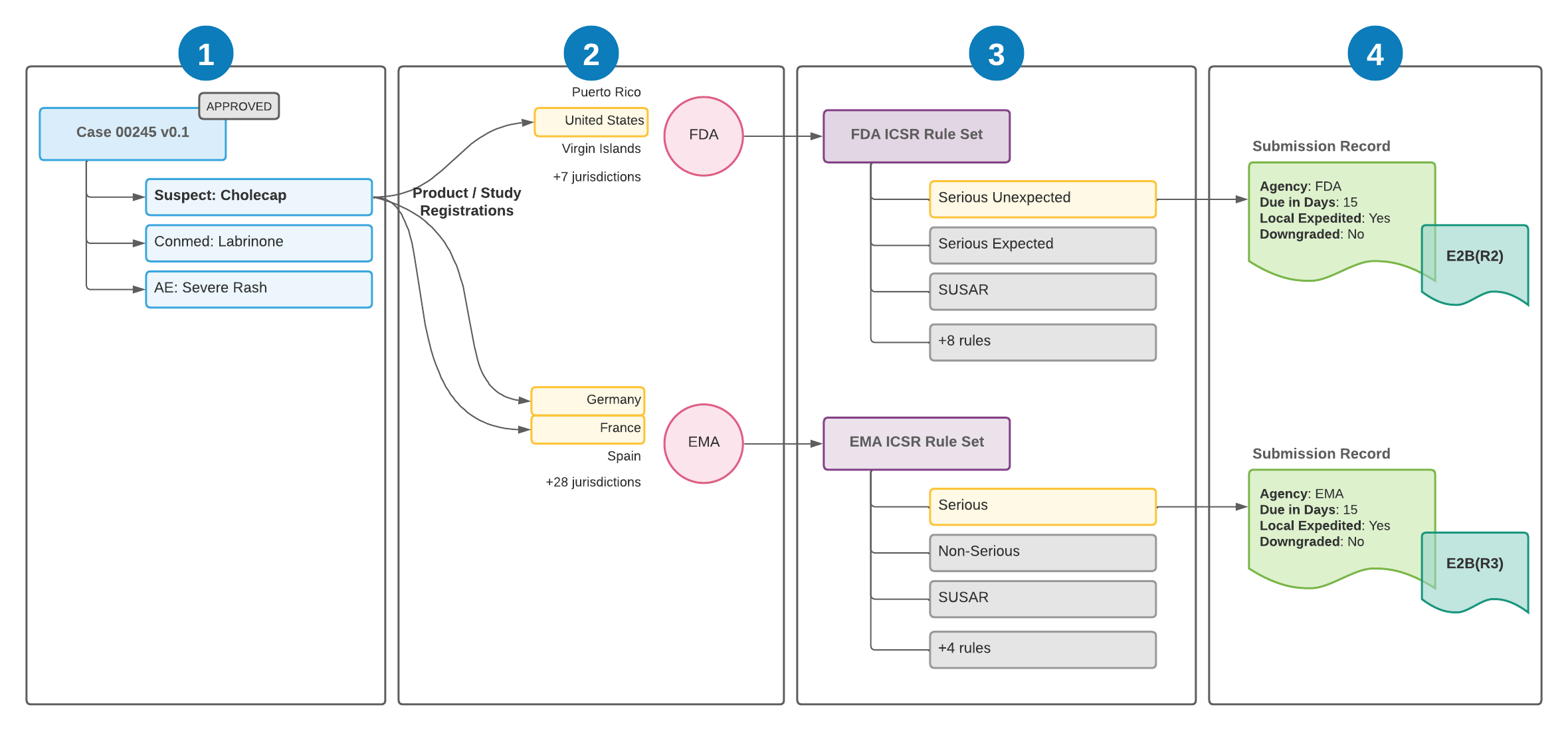
The following illustration shows how Vault Safety evaluates regulatory reporting requirements for general reporting:
![]() Case 00245 is entered and approved. The Case contains the primary Case Product of
Case 00245 is entered and approved. The Case contains the primary Case Product of Cholecap. The Case triggers the reporting rules engine by moving to the Approved state.
![]() To determine regulatory reporting requirements, the reporting rules engine evaluates Cholecap’s Product Registrations.
To determine regulatory reporting requirements, the reporting rules engine evaluates Cholecap’s Product Registrations.
Cholecap has Product Registrations for the following countries:
- United States: This country’s jurisdiction is the FDA. Vault proceeds to evaluate the FDA rule set to generate an FDA Submission.
- Germany: This country’s jurisdiction is the EMA. Vault proceeds to evaluate the EMA rule set to generate an EMA Submission.
- France: This country’s jurisdiction is also the EMA. No further Submissions are generated because another EMA registration is already being evaluated. Vault generates a single Submission when there are multiple registrations with the same agency jurisdiction.
![]() For each rule set, the rules engine iterates through the rules in order. Each rule consists of parameters that define how it is evaluated. Each parameter is evaluated against Case information. When all parameters of a rule are evaluated successfully, the rule is considered a match, and no other rules are evaluated for that rule set.
For each rule set, the rules engine iterates through the rules in order. Each rule consists of parameters that define how it is evaluated. Each parameter is evaluated against Case information. When all parameters of a rule are evaluated successfully, the rule is considered a match, and no other rules are evaluated for that rule set.
![]() Vault generates Submission records for the Case using the matching rules, and populates the due date and other fields on the Submission accordingly. Vault generates the transmission document in the appropriate format for submission.
Vault generates Submission records for the Case using the matching rules, and populates the due date and other fields on the Submission accordingly. Vault generates the transmission document in the appropriate format for submission.
Distribution Rules
Assign a Safety Rule Set to a Reporting Family to automatically generate Distributions for trading partners. Vault Safety supports the following types of Reporting Families for Distributions:
- Partner Distribution List: You can create a Partner Distribution List to automatically generate Distributions for partners or sites.
- MAH Distribution List: Assigned through the Reporting Organization field on a Product Registration or Study Registration, an MAH Distribution List generates a Distribution to a market authorization holder (MAH) in place of generating a Submission to an agency.
Note: In the event where both a Reporting Obligation and one or more Partner Distribution Rules exist for a single active Product Registration or Study Registration, Vault Safety creates a Distribution record for each reporting rule, including when both reporting rules are to the same destination.
Download the Submission Rule Log for a Case
When either of the Evaluate Reporting Obligations or Evaluate Early Reporting Obligations actions runs on a Case (whether manually or automatically as part of the Case workflow), Vault keeps a log of which Destinations were evaluated (Agencies or Partners for Early Notifications, Agencies for Submissions, Partners for Distributions). It also logs the names of any Transmission Output Templates used and whether the Case passed one (1) or more of the associated Destination’s safety rules.
You can access this log from the All Actions menu of a Case by selecting Download Submission Rule Log. The log is in CSV format. Once downloaded, you can open the file in a text editor or spreadsheet application.
Note: If you experience unexpected behavior when generating Transmissions, such as an Early Notification, a Submission, or a Distribution not generating when it should, your Admin can use the Troubleshooting Safety Rules article to help diagnose and fix the Safety Rules in your Vault.