Learn how to set up and generate CIOMS II reports.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
About CIOMS II Reports
Safety provides Council for International Organizations of Medical Sciences (CIOMS) II authoring and table generation capabilities. The Safety CIOMS II report adheres to the ICH E2C(R1) regulatory guideline. You can also generate reports in the CIOMS II line listing format using filters such as Report Type, Case Seriousness, and Case Expectedness that do not use PSUR regulations logic.
The following table summarizes the CIOMS II tabulations that Vault generates:
| Tabulation | Generated by Default? | Masking Support? |
|---|---|---|
| CIOMS II Interval Line Listing of Adverse Drug Reactions | Yes | Yes |
Note: Your Admin can configure custom CIOMS II report templates for your organization.
Prerequisites
Consider the following prerequisites before you generate CIOMS II tables:
- You must be assigned permissions to view and prepare aggregate reports. Typically, these permissions are reserved for the Safety Writer and Head of Safety roles.
- Your Admin must have enabled CIOMS II Line Listings.
- To filter reports by country as well as generate reports in the CIOMS II line listing format using filters unrelated to PSUR regulations logic, your Admin must also enable Additional Filters for CIOMS II Aggregate Report.
- The Reporting Family is set up with the Products, Studies, and Substances to include in the CIOMS II tabulations and CIOMS II line listings.
- Your Admin must have configured a Datasheet for each Product, Product Registration, Study, or Study Product with a list of expected adverse events for the reporting family.
- Vault uses these Datasheets to classify adverse events as listed or unlisted in CIOMS II tabulations.
- To add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report, your Admin must enable the Criteria Page for Aggregate reports.
Create a CIOMS II Aggregate Report
Create a CIOMS II Aggregate Report and specify the report settings.
Add a CIOMS II
- In the Vault primary navigation bar, select Aggregate Reports > CIOMS II and then select Create.
- In the Create Aggregate Report window, under Select Aggregate Report Type select CIOMS II.
- Complete the fields on the Create CIOMS II page.
- Populate the Original CIOMS II Logic field to specify what kind of logic you want the report to consider:
- Select Yes to generate a CIOMS II line listing report using PSUR regulations logic.
- Select No to generate reports in the CIOMS II line listing format using filters unrelated to PSUR regulations logic and to reveal additional filters.
- Populate the Original CIOMS II Logic field to specify what kind of logic you want the report to consider:
- (Optional) To limit the report to specific countries, add the applicable locations in the Country section.
- Save the record.
Result
The CIOMS II record enters the Pending state. Vault assigns a task to users in the Safety Writer role to review the report details.
CIOMS II Fields
You can specify the following fields for a CIOMS II Aggregate Report:
| Field | Description |
|---|---|
| Product Family (Required) | Select the Reporting Family configured for aggregate reporting.
Note: The Reporting Family object type should be Product Family.
|
| Organization | Vault populates this field with the Organization on the selected Reporting Family. |
| Data Period Start (Required) | Enter the start date for the reporting period. Vault uses the Cases within the reporting period to generate the table data. Cases are included when the date corresponding to the Filter Case By setting is within the reporting period. Cumulative reports do not consider the start date. The data period contains all Cases up to the Data Period End Date. To learn more, see How Aggregate Reports Filter by Data Period. |
| Data Period End (Required) | Enter the end date for the reporting period. To learn more, see How Aggregate Reports Filter by Data Period. |
| Filter Case By | To customize how Vault filters Cases within the specified date range, select an option:
If this field is blank, Vault uses the Case Receipt Date/New Info Date. |
| Include Criteria Page on Documents | Select the checkbox to add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report. When selected, the criteria page summarizes the following:
|
| States to Include (Required) | Select the states that Cases must be in to be included in the report. By default, only Cases in the Approved, Closed, Superseded, and Medical Review states are included. Note that while Superseded is not listed as an option, the Closed state includes the Superseded state. Only system-provided states in the Case Processing Lifecycle are supported.
Note: For Cases that have been nullified, ensure to change the Case Lifecycle State to a state that is not listed in the States to Include field. For example, if a nullified Case remains in the Closed state, Vault will still consider the Case in aggregate reports.
|
| Original CIOMS II Logic | Select Yes to generate the standard CIOMS II line listing report based on PSUR regulations to filter Cases. Select No to generate a variety of reports in the CIOMS II line listing format based on filters that do not consider PSUR logic. |
| Report Type | Select the report type to include in the report. This field appears when you select No for Original CIOMS II Logic. |
| Case Serious | Select Yes or No to generate Cases with matching values. This field appears when you select No for Original CIOMS II Logic. |
| Case Relatedness | Select the relatedness values to include in the report. This field appears when you select No for Original CIOMS II Logic. |
| Case Expectedness | Select the expectedness values to include in the report. This field appears when you select No for Original CIOMS II Logic. |
| Expectedness Source | Specify how the report calculates expectedness. Select Datasheets to calculate from Datasheets or Case to calculate from Case Assessments. This field appears when you select No for Original CIOMS II Logic. |
| Drug Roles to Include | Select one or more Drug Roles from the dropdown list to include in the Interval Line Listing of Adverse Drug Reactions tabulations. The Suspect and Interacting Drug Roles are selected by default for CIOMS II reports created with the 23R3 release or later. For CIOMS II reports created from prior releases, update the field to specify or revise which Drug Roles to include in the report. |
| Generate Masked Documents | Select this option to generate a masked copy of the Interval Line Listings of Adverse Drug Reactions table for masked distributions To learn more, see Generate Masked Aggregate Tabulations (CIOMS II, PBRER and DSUR). |
| Include Criteria Page on Documents | Select this option for the cover page to list the criteria used to generate the report. |
| Indicate Unexpected Term | Select Yes to display the unexpected adverse event term in the CIOMS II Line Listings. |
| Datasheet | This field works alongside the Indicate Unexpected Term setting for evaluating approved terms in product datasheets. You can specify the following options for CIOMS II reports:
Note:
|
| Comments | To customize the reports, select optional fields to be included in the report. Most of these will appear in the comments area of the report. You can select the following options:
|
| Countries | To filter by country, select Add in the Countries section to use the Search: Country dialog to specify the countries to include in the report. The report will include all Cases with a matching Reporter Country as well as all Cases with a matching Event Country for Case Adverse Events. |
| 1. This option requires the Allow Narrative Preview to be included in the report Aggregate Report setting to be enabled in your Vault. This setting is enabled by default. See the Narrative Preview Option for CIOMS II Reports for more information. | |
Generate CIOMS II Tabulations
Review and verify the report settings. Once you have confirmed the report details are correct, use the Generate Aggregate Report Tabulations action to generate CIOMS II report tables.
Mark Unexpected Terms in CIOMS II Reports
You can set the Indicate Unexpected Term on CIOMS II reports so that Vault marks each unexpected adverse event.
To identify unexpected events, your Admin must have configured a Datasheet for each Study and Product in the CIOMS II Reporting Family. Where they exist, Datasheets are evaluated by Vault in the following priority order:
Datasheets can specify the Active Date Start and, optionally, an Active Date End, which indicates when a term is approved as expected for the product. If configured, The CIOMS II Start Date must be within a term’s active range to be considered expected.
Expectedness in Aggregate Reports provides more information.
Note: If an active start date is defined for the term on the Datasheet, Vault considers the Active Range for Expectedness. This behavior always applies to CIOMS II, regardless of the Indicate Unexpected Term setting.
CIOMS II Table Generation Data Mapping
Vault populates aggregate report tables using Cases within the reporting period specified on the CIOMS II, and the reporting family members configured on the associated Reporting Family.
Note: For blinded studies, Vault populates blinded product information as Blinded in the generated tables. If the Blinded Placeholder Name field is populated then this will display on the report instead of the generic Blinded label.
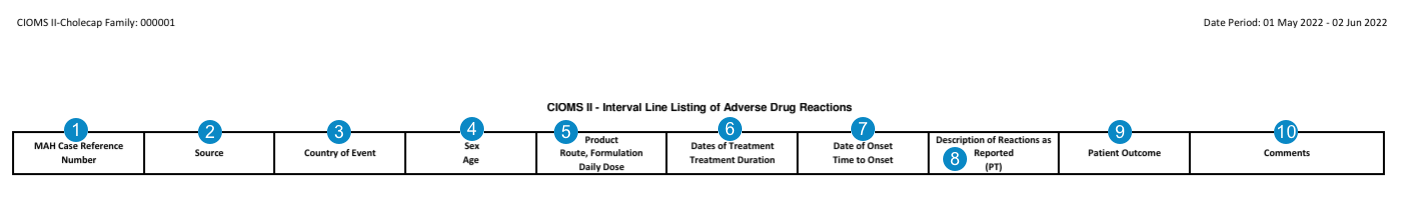
CIOMS II Interval Line Listing of Adverse Drug Reactions
Table Constraints
Vault filters Cases to include in the CIOMS II Interval Line Listing of Adverse Drug Reactions using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the CIOMS II.
case_version__v.state__v CONTAINS cioms_ii__v.states_to_include__v
Consider the folllowing:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
- Nullified (
Note: You cannot select these states in the States to Include field. These states are always omitted.
- If the Case is in a Lifecycle State assigned a State Type of “Deleted”, the Case is omitted.
- When evaluating the States to Include field, Vault evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
Case Date in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≥ cioms-ii__v.data_period_start__v AND
DATE ≤ cioms-ii__v.data_period_end__v
where DATE depends on the option selected in the CIOMS II Filter Cases By (cioms-ii__v.filter_cases_by__v) field:
- When Approval Date:
case_version__v.approval_date__v - When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v - Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
Suspect, Interacting, or Drug Not Administered Case Product or Substance in Reporting Family
A Case Product must meet both of the following conditions:
-
The Case Product must be a member of the Reporting Family
case_version__vr.case_product__v.product__v INaggregate_report_family__vr.aggregate_report_family_join__vr.products__vOR
The Case Product must have a Product Substance that is a member of the Reporting Familyreporting_family_v.substance__v.substance_v ≠ BLANKAND reporting_family_v.substance__v.substance_v =case_product__v.product__v.product_substance__v -
The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered
Case Report Type, Seriousness, Expectedness, and Causality
To generate reports using the original CIOMS II logic based on PSUR regulations, the Case must match one of the following scenarios:
| Scenario | Report Type | Seriousness | Expectedness | Causality Established |
|---|---|---|---|---|
| 1 |
|
Serious (not blank) | Any | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 1
AND case_version_v.seriousness__v ≠ blank |
||||
| 2 |
|
Blank (Non-Serious) | Unexpected | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 1
AND case_version_v.seriousness__v = blank
AND case_version_v.expected__v = false |
||||
| 3 |
|
Serious (not blank) | Any | For any primary Case Assessment:
|
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 2
AND case_version_v.seriousness__v ≠ blank
AND case_assessment__v.case_assessment_result__v.causality_established__v =
WHERE case_assessment__v.case_product__v.primary__v = True |
||||
| 4 |
|
Serious (not blank) | Any | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 3 OR 4
AND case_version_v.seriousness__v ≠ blank |
||||
| 5 |
|
Blank (Non-Serious) | Unexpected | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 3 OR 4
AND case_version_v.seriousness__v = blank
AND case_version_v.expected__v = false |
||||