Learn how to set up PSUR tabulations and CIOMS II line listings
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
About PSUR Reports
Vault Safety provides Periodic Safety Update Report (PSUR) authoring and table generation capabilities, including CIOMS II line listings. The Vault Safety PSUR report adheres to the ICH E2C (R1) regulatory guideline.
Note: See Create CIOMS II Reports if you need to create a standalone CIOMS II report. The CIOMS II standalone report supports masking and can optionally include additional information beyond that required by regulations.
The following table summarizes the PSUR tabulations that Vault Safety generates:
| Tabulation | Generated by Default? | Masking Support? |
|---|---|---|
| CIOMS II Interval Line Listing of Adverse Drug Reactions | Yes | No |
| Summary Tabulation of Serious Unlisted Adverse Drug Reactions | Yes | No |
| Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions | Yes | No |
Note: An Admin can configure custom PSUR report templates for your organization.
Prerequisites
Consider the following prerequisites before you generate PSUR tables:
- You must be assigned permissions to view and prepare aggregate reports. Typically, these permissions are reserved for the Safety Writer and Head of Safety roles.
- An Admin must have enabled PSUR Tabulations and CIOMS II Line Listings
- The Reporting Family with the Products, Studies, and Substances to include in the PSUR tabulations and CIOMS II line listings.
- Depending on your Admin’s configuration, Case Products with the Drug Role of Drug Not Administered may be included when generating PSUR and CIOMS II Line Listing reports. See Enable Extend Definition of Suspect to Drug Not Administered for more information. If your Vault is not configured to include Drug Not Administered, only Case Products with the Suspect or Interacting Drug Role are included.
- An Admin must have configured each product’s Core Datasheet with a list of expected adverse events for the reporting family product. Vault Safety uses Core Product Datasheets to classify adverse events as listed or unlisted in PSUR tabulations.
- To add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report, your Admin must enable the Criteria Page for Aggregate reports.
Create a PSUR Aggregate Report
Create a PSUR Aggregate Report and specify the report settings.
Add a PSUR
- In the Vault primary navigation bar, select Aggregate Reports > PSUR, and then select Create. If you do not see PSUR as an option, an Admin must update your Vault to enable PSUR.
- In the Create Aggregate Report window, under Select Aggregate Report Type, select PSUR.
- Complete the fields on the Create PSUR page.
- Save the record.
Result
The PSUR record enters the Pending state. Vault assigns a task to users in the Safety Writer role to review the report details.
PSUR Fields
You can specify the following fields for a PSUR Aggregate Report:
| Field | Description |
|---|---|
| Product Family (Required) | Select the Reporting Family configured for aggregate reporting.
Note: The Reporting Family object type should be Product Family.
|
| Organization | Vault populates this field with the Organization on the selected Reporting Family. |
| Data Period Start (Required) | Enter the start date for the reporting period. Vault uses the Cases within the reporting period to generate the table data. Cases are included when the date corresponding to the Filter Case By setting is within the reporting period. Cumulative reports do not consider the start date. The data period contains all Cases up to the Data Period End Date. To learn more, see How Aggregate Reports Filter by Data Period. |
| Data Period End (Required) | Enter the end date for the reporting period. To learn more, see How Aggregate Reports Filter by Data Period. |
| Filter Case By | To customize how Vault filters Cases within the specified date range, select an option:
If this field is blank, Vault uses the Case Receipt Date/New Info Date. |
| Include Criteria Page on Documents | Select the checkbox to add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report. When selected, the criteria page summarizes the following:
|
| States to Include (Required) |
Select the states that Cases must be in to be included in the report. By default, only Cases in the Approved, Closed, Superseded, and Medical Review states are included. Although Superseded is not listed as an option, it is included within the Closed state. Only system-provided states in the Case Processing lifecycle are supported.
Note: If the latest Case version within the aggregate reporting period is in the Nullified or Voided state or in a lifecycle state assigned to the Deleted state type, the Case is excluded from the aggregate report.
|
| Indicate Unexpected Term | Select Yes to display an asterisk beside each unexpected adverse event term in the CIOMS II Line Listings. |
| Datasheet | This field works alongside the Indicate Unexpected Term setting for evaluating approved terms in product datasheets. You can specify the following options for PSUR reports:
|
Generate PSUR Tabulations
Review and verify the report settings. Once you have confirmed the report details are correct, use the Generate Aggregate Report Tabulations action to generate PSUR report tables.
Mark Unexpected Terms in PSUR Reports
You can set the Indicate Unexpected Term on a PSUR so that when the CIOMS II Line Listings are generated, Vault marks each unexpected adverse event with an asterisk (*).
To identify unexpected events, an Admin must have configured the Core Datasheet for the products in the PSUR Reporting Family.
The Datasheet can specify the Active Date Start, and optionally an Active Date End, which indicates when a term is approved as expected for the product. If configured, The PSUR Start Date must be within a term’s active range to be considered expected. For more information about how to define an active range for expectedness in aggregate reports, see Manage Datasheets and Auto-Expectedness.
Note: If an active start date is defined for the term on the datasheet, Vault considers the Active Range for Expectedness. This behavior always applies to PSUR, regardless of the Indicate Unexpected Term setting.
PSUR Table Generation Data Mapping
Vault Safety populates aggregate report tables using Cases within the reporting period specified on the PSUR, and the reporting family members configured on the associated Reporting Family.
Note: For blinded studies, Vault populates blinded product information as Blinded in the generated tables.
The following sections describe how Vault Safety generates PSUR tabulations:
- CIOMS II Interval Line Listing of Adverse Drug Reactions
- Summary Tabulation of Serious Unlisted Adverse Drug Reactions
- Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions
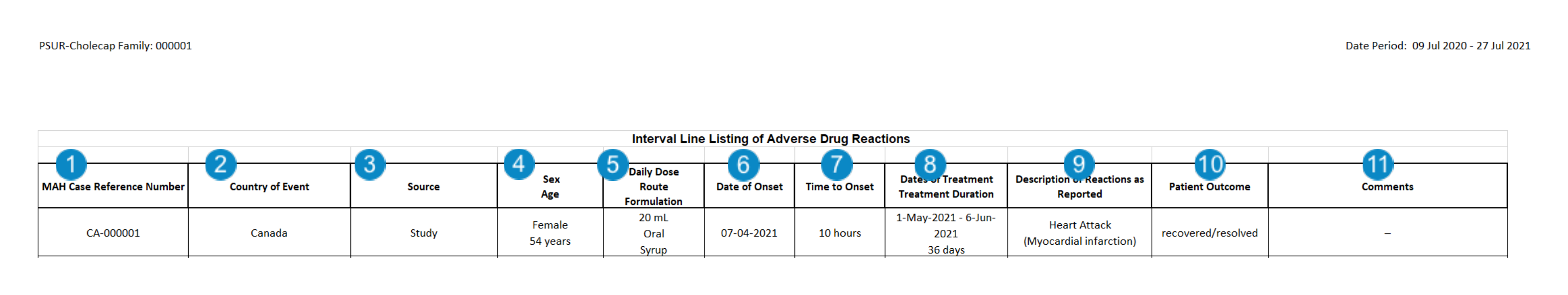
CIOMS II Interval Line Listing of Adverse Drug Reactions
Table Constraints
Vault filters Cases to include in the CIOMS II Interval Line Listing of Adverse Drug Reactions using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the PSUR.
case_version__v.state__v CONTAINS psur__v.states_to_include__v
Note the following considerations:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
- Nullified (
Note: You cannot select these states in the States to Include field. These states are always omitted.
- If the Case is in a Lifecycle State assigned a State Type of “Deleted”, the Case is omitted.
- When evaluating the States to Include field, the system evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
Case Date in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≥ psur__v.data_period_start__v AND
DATE ≤ psur__v.data_period_end__v
where DATE depends on the option selected in the PSUR Filter Cases By (psur__v.filter_cases_by__v) field:
- When Approval Date:
case_version__v.approval_date__v - When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v - Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
Suspect, Interacting, or Drug Not Administered Case Product or Substance in Reporting Family
A Case Product must meet both of the following conditions:
-
The Case Product must be a member of the Reporting Family
case_version__vr.case_product__v.product__v INaggregate_report_family__vr.aggregate_report_family_join__vr.products__vOR
The Case Product must have a Product Substance that is a member of the Reporting Familyreporting_family_v.substance__v.substance_v ≠ BLANKAND reporting_family_v.substance__v.substance_v =case_product__v.product__v.product_substance__v -
The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered
Case Report Type, Seriousness, Expectedness, and Causality
The Case must match one of the following scenarios:
| Scenario | Report Type | Seriousness | Expectedness | Causality Established |
|---|---|---|---|---|
| 1 |
|
Serious (not blank) | Any | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 1
|
||||
| 2 |
|
Blank (Non-Serious) | Unexpected | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 1
|
||||
| 3 |
|
Serious (not blank) | Any | For any primary Case Assessment:
|
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 2
|
||||
| 4 |
|
Serious (not blank) | Any | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 3 OR 4
|
||||
| 5 |
|
Blank (Non-Serious) | Unexpected | Any |
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v = 3 OR 4
|
||||
Table Mapping
| Number | Name | Description |
|---|---|---|
| MAH Case Reference Number | The value from the Case UID field.case_version__v.uid__v |
|
| Country of Event | The value from the Case Event Country field.case_version__v.event_country__vr.name__v |
|
| Source | The value selected in the Case Report Type field.case_version_v.report_type__v |
|
| Sex Age |
Values from the following fields:
|
|
| Daily Dose, Route, Formation of Suspect Drug | The following information is listed:
If there are multiple Dosages under the primary Case Product, values from each Dosage record are displayed in a line-separated list. |
|
| Date of Onset | The date entered in the Date of Onset field on the primary Case Adverse Event in the format DD-MM-YYYY.case_adverse_event__v.onset_date__v |
|
| Time to Onset | The value from the First Dose Latency on the primary Case Adverse Event.case_assessment__v.first_dose_interval_number__v |
|
| Dates of Treatment Treatment Duration |
Values are mapped from primary Case Product Dosages as follows:
|
|
| Description of Reaction Reported | The list of adverse events, including both the reported term and MedDRA preferred term from each Case Adverse Event.
Data from the primary Case Adverse Event is listed first, followed by other Case Adverse Events ordered by rank. |
|
| Outcome | The value selected in the Case Adverse Event Outcome field. If there are multiple Case Adverse Event records on a Case, Vault displays the outcome of the primary Adverse Event (normally the most serious Adverse Event on the Case). case_adverse_event__v.event_outcomes__v.name__v
|
|
| Comments |
Any text entered in the Case Reporting Summary field. You can use this field to highlight causality information.case_version__v.reporting_summary__v
|
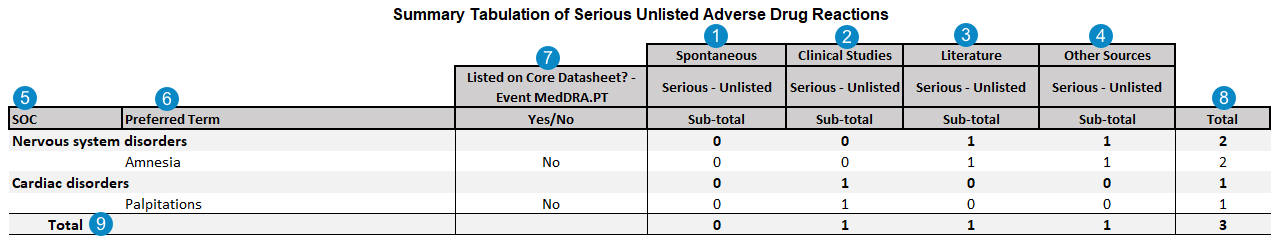
Summary Tabulation of Serious Unlisted Adverse Drug Reactions
The following image map shows how Vault Safety generates the PSUR Summary Tabulation of Serious Unlisted Adverse Drug Reactions table.
Note: The table above displays the Totals when the PSUR Summary Totals and Separate Log Files feature is enabled. Contact your Admin if you want this feature configured in your Vault.
Table Constraints
Vault filters Cases to include in the Summary Tabulation of Serious Unlisted Adverse Drug Reactions using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
Suspect, Interacting, or Drug Not Administered Case Product or Substance in Reporting Family
A Case Product must meet both of the following conditions:
-
The Case Product must be a member of the Reporting Family
case_version__vr.case_product__v.product__v INaggregate_report_family__vr.aggregate_report_family_join__vr.products__vOR
The Case Product must have a Product Substance that is a member of the Reporting Familyreporting_family_v.substance__v.substance_v ≠ BLANKAND reporting_family_v.substance__v.substance_v =case_product__v.product__v.product_substance__v -
The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered
Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the PSUR.
case_version__v.state__v CONTAINS psur__v.states_to_include__v
Note the following considerations:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
- Nullified (
Note: You cannot select these states in the States to Include field. These states are always omitted.
- If the Case is in a Lifecycle State assigned a State Type of “Deleted”, the Case is omitted.
- When evaluating the States to Include field, the system evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
Case Date in Cumulative Reporting Period
The date must be within the aggregate report cumulative reporting period (Product IBD to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≤ psur__v.data_period_end__v
where DATE depends on the option selected in the PSUR Filter Case By (psur__v.filter_cases_by__v) field:
- When Approval Date:
case_version__v.approval_date__v - When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v - Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, only the most recent Case version within the reporting period is listed.
Table Mapping
| Number | Name | Description | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spontaneous Serious - Unlisted Sub-total |
Number of adverse events that meet the following criteria:
|
||||||||||||||
| Clinical Studies Serious - Unlisted Sub-total |
Number of adverse events that meet the following criteria:
|
||||||||||||||
| Literature Serious - Unlisted Sub-total |
Number of adverse events that meet the following criteria:
|
||||||||||||||
| Other Sources Serious - Unlisted Sub-total |
Number of adverse events that meet the following criteria:
|
||||||||||||||
| SOC | The MedDRA System Organ Class (SOC) for the adverse event. If the MedDRA term is not coded on the Case Adverse Event, the SOC is blank.
event_meddra__v.soc_term__v |
||||||||||||||
| Preferred Term | The MedDRA Preferred Term for the adverse event.case_adverse_event__v.event_meddra__v.pt_term__vNote: Contact Veeva Support to request PT Aggregation in periodic reports, which counts only unique instances of Preferred Terms (PT) in summary tabulations. Once this feature is enabled, when a Case contains multiple Case Adverse Events coded under the same MedDRA Preferred Term (PT), the report counts a single PT event instead of multiple events. |
||||||||||||||
| Listed on Core Datasheet? - Event MedDRA.PT Yes/No |
Whether each adverse event is listed on the Core Datasheet for the primary Case Product within the reporting period. Vault uses the MedDRA Preferred Term (PT) to determine whether an adverse event is listed. If an active start date is defined for the term on the datasheet, Vault considers the Active Range for Expectedness. Yes IF
|
||||||||||||||
| Total | The sum of the Spontaneous, Clinical Studies, Literature, and Other Sources occurrences for each SOC and Preferred Term. | ||||||||||||||
| Total | The total number of Serious Unlisted Adverse Drug Reaction occurrences for each of the Spontaneous, Clinical Studies, Literature, and Other Sources categories. | ||||||||||||||
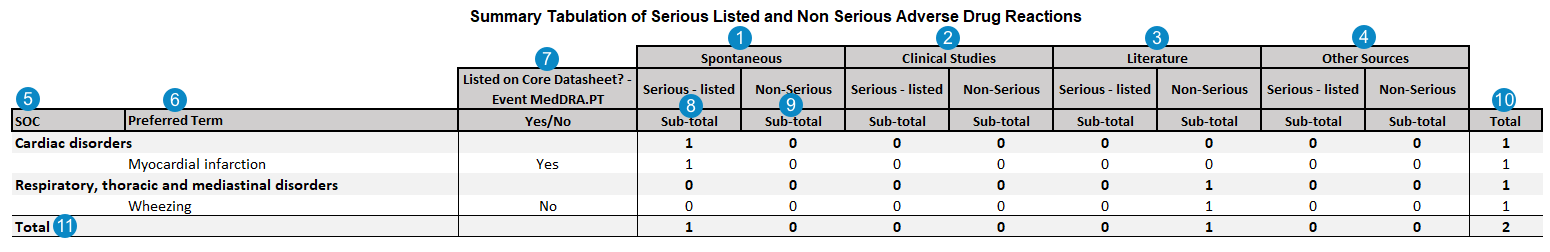
Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions
The following image map shows how Vault Safety generates the PSUR Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions table.
Note: The table above displays the Totals when the PSUR Summary Totals and Separate Log Files feature is enabled. Contact your Admin if you want this feature configured in your Vault.
Table Constraints
Vault filters Cases to include in the Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
Suspect, Interacting, or Drug Not Administered Case Product or Substance in Reporting Family
A Case Product must meet both of the following conditions:
-
The Case Product must be a member of the Reporting Family
case_version__vr.case_product__v.product__v INaggregate_report_family__vr.aggregate_report_family_join__vr.products__vOR
The Case Product must have a Product Substance that is a member of the Reporting Familyreporting_family_v.substance__v.substance_v ≠ BLANKAND reporting_family_v.substance__v.substance_v =case_product__v.product__v.product_substance__v -
The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered
Case Date in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≥ psur__v.data_period_start__v AND
DATE ≤ psur__v.data_period_end__v
where DATE depends on the option selected in the PSUR Filter Cases By (psur__v.filter_cases_by__v) field:
- When Approval Date:
case_version__v.approval_date__v - When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v - Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, only the most recent Case version within the reporting period is listed.
Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the PSUR.
case_version__v.state__v CONTAINS psur__v.states_to_include__v
Note the following considerations:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
- Nullified (
Note: You cannot select these states in the States to Include field. These states are always omitted.
- If the Case is in a Lifecycle State assigned a State Type of “Deleted”, the Case is omitted.
- When evaluating the States to Include field, the system evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
Table Mapping
| Number | Name | Description | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spontaneous | Adverse events with the Case Report Type set to Spontaneous.case_version__v.report_type__v.controlled_vocabulary__v.e2b_code__v = 1
|
||||||||||||||
| Clinical Studies | Adverse events with the Case Report Type set to Study and the Study Type is set to Clinical Trial. This does include the Study (Literature) Report Type.case_version__v.report_type__v.controlled_vocabulary__v.e2b_code__v = 2 (Study)
|
||||||||||||||
| Literature | Number of adverse events with the Case Report Type set to one of the following:
|
||||||||||||||
| Other | An adverse event is counted when either of the following conditions are met:
|
||||||||||||||
| SOC | The MedDRA System Organ Class (SOC) for the adverse event. If the MedDRA term is not coded on the Case Adverse Event, the SOC is blank. event_meddra__v.soc_term__v |
||||||||||||||
| Preferred Term | The MedDRA Preferred Term for the adverse event.case_adverse_event__v.event_meddra__v.pt_term__v
Note: Contact Veeva Support to request PT Aggregation in periodic reports, which counts only unique instances of Preferred Terms (PT) in summary tabulations. Once this feature is enabled, when a Case contains multiple Case Adverse Events coded under the same MedDRA Preferred Term (PT), the report counts a single PT event instead of multiple events. |
||||||||||||||
| Listed on Core Datasheet? - Event MedDRA.PT Yes/No |
Whether each adverse event is listed on the Core Datasheet for the primary Case Product. Vault uses the MedDRA Preferred Term (PT) to determine whether an adverse event is listed. If an active start date is defined for the term on the datasheet, Vault considers the Active Range for Expectedness. Yes IF
|
||||||||||||||
| Serious - listed Sub-total |
Number of adverse events that meet the following criteria:
|
||||||||||||||
| Non-Serious | Number of adverse events that do not have a value selected in the Case Adverse Event > Seriousness field.case_adverse_event__v.seriousness__v = EMPTY |
||||||||||||||
| Total | The sum of the Spontaneous, Clinical Studies, Literature, and Other Sources occurrences (both Serious - listed and Non-Serious) for each SOC and Preferred Term. | ||||||||||||||
| Total |
For each of the Spontaneous, Clinical Studies, Literature, and Other Sources categories:
|
||||||||||||||
With the PSUR Summary Totals and Separate Log Files feature enabled in your Vault, a separate log file lists the Cases included in the Summary Tabulation of Serious Unlisted Adverse Drug Reactions and Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions. The log file uses the Aggregate Reports > Summary Tabulation > Log document type.
Without the PSUR Summary Totals and Separate Log Files feature enabled in your Vault, the Cases included in the Summary Tabulation of Serious Unlisted Adverse Drug Reactions and Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions are listed in a separate table as part of the report.