Learn about features that support the intake and processing of drug cases for Japan and reporting to the PMDA.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
About Intake and Case Processing for the PMDA
Vault supports the intake and processing of postmarketing and investigational drug cases that originate within and outside of Japan. This includes the following features:
- Text translation through automation or dual-entry fields
- Code terms using industry-standard dictionaries
- Capture of data points specific to PMDA reporting, including clinical trial requirements for foreign cases
- Import of PMDA E2B(R3) files to Inbox Items for creating Japan Domestic Cases
- Calculation of reportable product registrations, localized assessments, and due dates
- Generation of local reporting details, comments, and Submissions
- Calculation of Japan reportability
For information on configuring all available capabilities on this page, see Prerequisites in PMDA Overview and Profile Setup.
Japan Case Intake
Vault supports the intake of drug cases reportable to Japan through Inbox Items. For general information on this topic, see Perform Local Language to English Intake.
You can complete intake for the following types of cases:
- Domestic Cases: Cases reported from within Japan
- To create a Domestic Case for Japan, set the Inbox Item Localization field to “Japanese (Japan)”.
- Localized Cases: Foreign cases reported from outside of Japan
- After the global Case is approved, Vault generates a Localized Case for Japan when the Case includes a Case Product Registration that is reportable to the PMDA.
For PMDA special report classifications, you can identify Safety Measure and Research Reports on Inbox Items.
Vault also supports importing PMDA E2B(R3) files to create Inbox Items for Japan domestic Cases, including merging to an in-flight Case and promoting to a follow-up Case through the Inbox Item to Case Compare page. For more information, see PMDA E2B(R3) Case Import.
Note: Although Vault supports the PMDA E2B(R3) data elements in section J2, these fields are not available on the Inbox Item during manual creation. You must enter the required data after promoting the Inbox Item to a Case.
Translating Inbox Items and Cases
When you save an Inbox Item with the Localization field set to Japanese (Japan), you can use the local intake fields to enter information in both Japanese and English. These dual-entry fields also appear on the Japan domestic Case after promotion.
For foreign cases, enter details in Japanese on the Localized Case.
If your Admin has set up the Auto-Translation Framework, you can send all localized text fields for Inbox Items or Cases to Amazon Translate for translation into English.
Code Inbox Item and Case Terms
Vault supports Japanese coding of terms on Inbox Items and Cases using industry-standard dictionaries, as follows:
- Code medical terms for symptoms, diseases, indicators, and adverse events using the multilingual Medical Dictionary for Regulatory Activities (MedDRA). For more information about the multilingual MedDRA Browser, see Code MedDRA Terms.
- This includes both current and J-Current terms, enabling coding different English terms that translate to the same Japanese characters
- The MedDRA language is set based on the Localization field
- Code external products using the Japan Drug Dictionary (JDD). For more information, see Code Japan Drug Dictionary Products.
- Code external products or add drug history to the Case using the WHODrug Dictionary. For more information, see Code WHODrug Products.
Cross-Referencing JDrug and WHO Drug Codes
Vault also supports cross-referencing JDrug and WHODrug codes during case processing using the WHODrug Cross Reference Tool (CRT) Japan. This eliminates manual code entry in the following instances:
- Adding the applicable WHODrug code when processing a Japan domestic Case with an External Product coded using a JDrug code.
- Adding the applicable JDrug code when processing a Japan foreign Localized Case with an External Product coded using a WHODrug code.
For more information on using this feature, see Code Japan Drug Dictionary Products and Code WHODrug Products.
Case Processing Overview
After you promote the Inbox Item to a domestic Case or Vault generates a Localized Case, you can complete additional data entry, medical review, and Case validation.
Domestic Cases and Localized Cases support region-specific fields for reporting to the PMDA. For general information on domestic Cases, see Prepare a Domestic Case. For general information on Localized Cases, see Prepare a Localized Case.
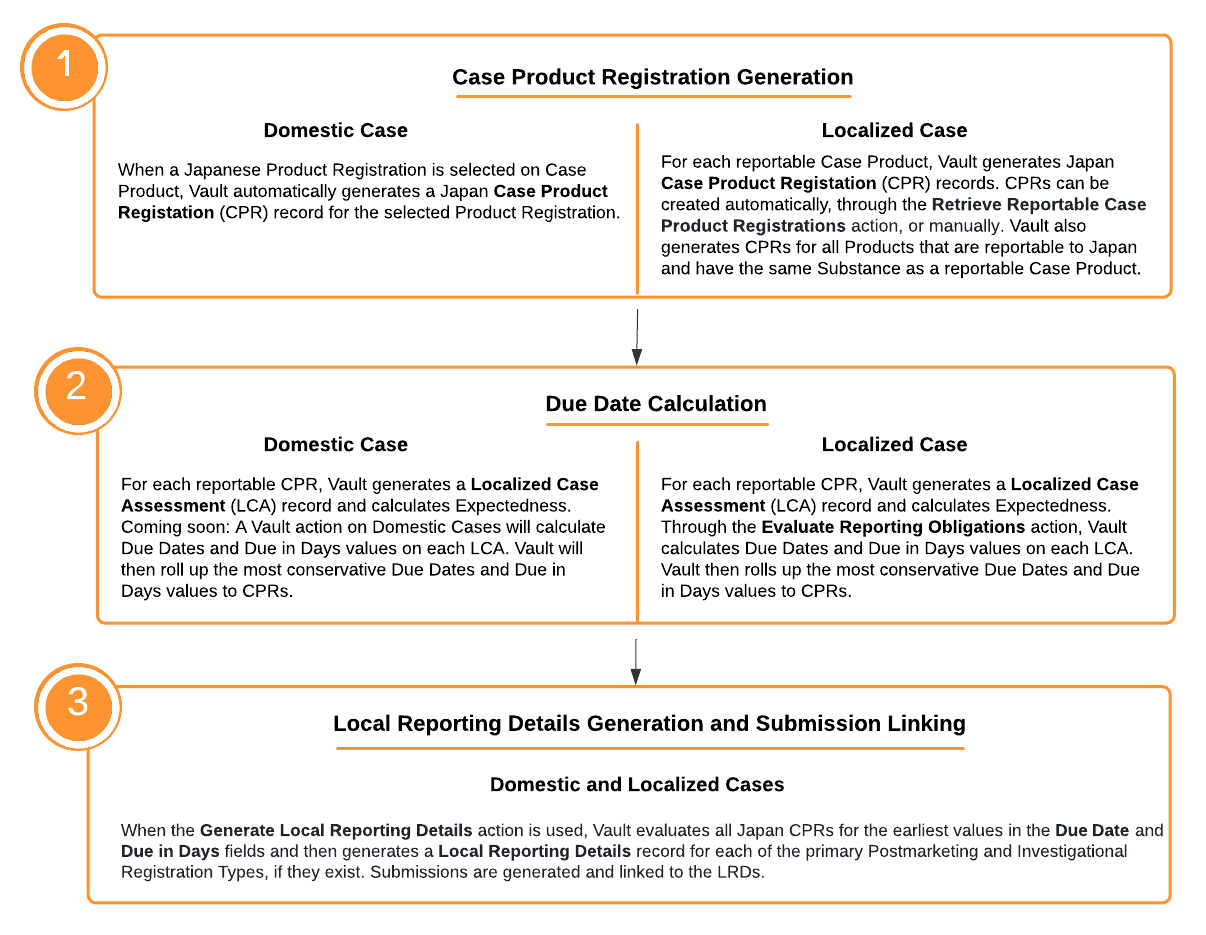
The following diagram highlights the major Case processing steps that enable Vault to determine reporting requirements for the PMDA.
Calculate Japan Reportability
The Calculate Japan Reportability action on Japan Localized Cases generates Case Product Registration records, evaluates their reporting obligations, and creates the associated Local Reporting Details records and Submissions.
Case Product Registration Section
The Case Product Registration section captures Japan Case Product Registrations. For both domestic Cases and Localized Cases, during Case Product Registration record creation, details are mapped from the Japan Product Registration when available. See the sections below for information on when Vault generates records for Domestic Cases and Localized Cases. For information on the available Case Product Registration fields, see PMDA Case Field Reference
Case Product Registration and Assessment records are not generated for Device or OTC-Device Case Products or Device Constituents of Combination Products.
When you delete Case Product Registrations, Vault cascades deletions to associated localized records and clear references in related records.
Domestic Case Product Registration Generation
On domestic Cases for Japan, when a PMDA Product Registration is selected for the Case Product, Vault populates Case Product Registrations and the associated Case Assessments. Vault generates Case Product Registrations and Case Assessments in the following scenarios:
- Upon Case promotion if a PMDA Product Registration exists on the Inbox Item
- Upon using the Re-generate Domestic Case action
- Upon adding a Case Product with a PMDA Product Registration with an eligible Drug Role
- Upon changing the Product Registration of an existing Case Product with an eligible Drug Role from a blank or non-PMDA Product Registration to a PMDA Product Registration
- Upon changing the PMDA Product Registration of an existing Case Product with an eligible Drug Role to a different PMDA Product Registration
- Upon using the Evaluate Regulatory Conformance action
Vault deletes Case Product Registrations and associated Case Assessments upon changing the Product Registration of an existing Case Product with an eligible Drug Role from a PMDA Product Registration to either a non-PMDA Product Registration or to blank. If your Admin has enabled Isolate Blinded Clinical Trial Information in your Vault, Vault deletes Case Product Registrations in this scenario only when the Blinding Type is not Blinded.
Localized Case Product Registration Generation
Depending on your Admin’s configuration, Vault may generate Case Product Registrations upon Localized Case creation. To manually generate Case Product Registrations, from the All Actions menu, select Retrieve Reportable Case Product Registrations. When you run this action, Vault:
- Deletes both Vault-generated and manually created Case Product Registrations
- Generates new Case Product Registrations based on the latest Case Product updates
- Clears the Primary Case Product Registration field on Local Reporting Details
- For Cases related to an unknown formulation Product, Vault generates Localized Case Product Registrations for each Product Registration within the Product Family that is related to the unknown formulation Product
For blinded Study Products, Vault generates Case Product Registrations using the following priority:
- The primary blinded investigational product identified on the Study Product Placeholder record
- The earliest created Study Product with a Study Product Role of Investigational that is associated with the Study Product Placeholder record
- The earliest created Study Arm Product with an associated Study Product that has a Study Product Role of Investigational
When populating reportable Case Product Registrations, Vault evaluates all reporting obligations in the following instances:
- Marketing Registrations (General Reporting)
- Marketing Registrations (Cross Reporting)
- Investigational Registrations (General Reporting)
- Investigational Registrations (Cross Reporting)
- A product reportable to the PMDA includes the same substance as a suspect or interacting Case Product on the Localized Case (see both Cross Reporting scenarios linked above)
- A blinded Study Product is reportable to the PMDA
Vault considers existing Case Product Registrations not reportable and removes them from domestic Cases or Localized Cases in the following instances:
- The Drug Role of the associated Case Product is no longer Suspect, Interacting, or Drug Not Administered.
- If your Admin has enabled Isolate Blinded Clinical Trial Information in your Vault, Vault deletes Case Product Registrations in this scenario only when the Blinding Type is not Blinded.
- The Product or Product Registration associated with the Case Product or Study Product was deleted, inactivated, or deprecated.
Marketing Registrations (General Reporting)
The Japanese Registration Category is Marketing and the following details from the Localized Case Product match a Case Product Registration:
- Dose Form, Strength Number, and Strength Unit
If the above don’t all match, Vault matches on Dose Form alone. - The PMDA Product Registration is set up as the default registration
If there are multiple matches, Vault selects the PMDA Product Registration with a matching Dose Form and the earliest Created Date.
Marketing Registrations (Cross Reporting)
If Vault did not create a Marketing Case Product Registration for general reporting, then Vault generates the following Case Product Registrations for each Case Product for cross reporting:
- One (1) Case Product Registration from the Marketing Registrations for the Case Product using the same selection criteria as for Marketing Registrations (General Reporting).
- For the exact Substance-matched cross-reportable Products, a Case Product Registration for one (1) registration per exact matching cross-reportable Product using the same selection criteria as for Marketing Registrations (General Reporting).
- If there are no exact Substance-matched cross-reportable Products, then for each partial Substance-matched cross-reportable Product, Vault generates a Case Product Registration for one (1) registration per partial matching cross-reportable Product using the same selection criteria as for Marketing Registrations (General Reporting).
Investigational Registrations (General Reporting)
The Japanese Registration Category is Investigational.
If there are multiple matches, Vault adds them all to the Localized Case.
Investigational Registrations (Cross Reporting)
For Investigational Registrations registered to the PMDA, Vault generates a Case Product Registration for cross reporting if it is a Registration for a Product on the Case or a Registration for a cross-reportable Product with exact matching Substances.
Local Reporting Details Section
If your Admin has enabled the PMDA Local Reporting Details Generation and Submission Linking feature, you can select the Generate Local Reporting Details action in the All Actions menu on Japan Domestic and Localized Cases. For all Japan Case Product Registrations with values in the Due Date and Due in Days fields, Vault generates a Local Reporting Details record for each of the primary Postmarketing and Investigational Registration Types, if they exist on the Case. For information on the available Local Reporting Details fields, see PMDA Case Field Reference.
Vault determines the primary Case Product Registration using the following priority order:
- The earliest Due Date for each Registration Type, for example, Postmarketing or Investigational.
- If there are multiple registrations for a Registration Type with the same Due Date, the highest Rank is evaluated.
- The evaluation depends on the Registration Type:
- Postmarketing: If there are multiple registrations with the same Rank, the earliest Created Date is evaluated.
- Investigational: If there are multiple registrations with the same Rank, the CPR linked to a Study Product or Product on the Case is evaluated. If there are still multiple, the earliest Created Date is evaluated.
In addition to generating Local Reporting Details records, the Generate Local Reporting Details action generates records as follows:
- PMDA Reportable Products records as described in PMDA Reportable Products Section.
- Case Comments records as described in Case Comments Section.
- Submission records as described in Submission Generation and Linking for PMDA Localized Cases.
Vault considers Case Product Registrations without Due Date or Due in Days values to be not reportable.
Note: If your Admin has configured Isolate Blinded Clinical Trial Information in your Vault, the following occurs when you run the Generate Local Reporting Details action. If a Case is unblinded and includes unblinded and blinded Case Product Registrations, Vault sets the unblinded Case Product Registration on the related Local Reporting Details record. In addition, Vault generates one (1) PMDA Reportable Products record for each of the unblinded Case Product Registrations.
PMDA Reportable Products Section
To generate the PMDA Reportable Products section, which includes Local Reporting Details/Product Join object records, from the All Actions menu, select Generate Local Reporting Details. Local Reporting Details/Product Join records associate Local Reporting Details records with the reportable Products of the Case Product or Case Product Registration. For information on the available PMDA Reportable Products fields, see PMDA Case Field Reference.
In the PMDA Reportable Products section, Ranks are assigned by Registration Category and Drug Role as follows:
- For Marketing registrations, Vault assigns Rank 1 to the primary Case Product Registration from the Local Reporting Details section. Vault then ranks Other Postmarketing registrations in the following order based on Drug Role:
- Suspect
- Interacting
- Other
- For Investigational registrations, Vault assigns Rank 1 to the Case Product Registration with a Drug Role of Suspect. Vault then ranks in order based on Drug Role, including:
- Suspect
- Interacting
- Other
- For Investigational registrations with the same Drug Role, Vault assigns Rank based on the Rank in the Case Product Registration section.
Note: If Local Reporting Details are system-generated, Vault assigns a Rank based on the Rank in the Case Product Registration section.
Case Comments Section
The Case Comments section is generated through the Generate Local Reporting Details action and Localized Case Comment records are linked to their associated Local Reporting Details record. Use the Case Comments section to add PMDA (Japan) region-specific comments. For information on the available Case Comments fields, see PMDA Case Field Reference.
Adverse Events Section
Each Case Adverse Event from the Global Case is mapped to create Localized Case Adverse Events. For information on the available Adverse Event fields, see PMDA Case Field Reference.
Assessments Section
Vault supports generating Localized Case Assessment records for each Case Product Registration. To use this option, your Admin must turn on Localized Assessments for Case Product Registrations under Assessment Generation for the related Localization record. When turned on, for Suspect, Interacting, or Drug Not Administered Case Products, Vault generates Localized Case Assessment records and calculates Expectedness for each Case Product Registration.
With this feature, Vault generates Localized Case Assessments when you add Adverse Events to Cases. When you recode existing Adverse Events to a different MedDRA LLT, Vault updates Localized Case Assessments and reevaluates Expectedness. If your Admin has enabled Datasheet Expectedness by Age and Sex, Vault also re-evaluates Expectedness if you change the sex or any age-related field (including Date of Birth, Age at Onset, or Age Group) of the Case Patient.
For information on the available Assessment fields, see PMDA Case Field Reference.
Assessment records are not generated for Device or OTC-Device Case Products or Device Constituents of Combination Products.
Evaluate Reporting Obligations for Localized Case Assessments
Before evaluating general and cross reporting obligations, ensure you have retrieved all the reportable Case Product Registrations. For more information, see Case Product Registration Section.
If your Admin has not configured your Vault to generate Localized Case Assessments for Case Product Registrations, see Prepare a Localized Case for more information.
You can evaluate reporting obligations for Localized Case Assessments in the following ways:
- On a Japan Domestic Case, go to the All Actions menu and select Calculate Due Dates on LCA.
- On a Japan Localized Case, go to the All Actions menu and select Evaluate Reporting Obligations.
After running the applicable action to evaluate reporting obligations on the Localized Case, Vault populates the Due Date and Due in Days fields on each Localized Case Assessment as follows:
- Due in Days: Based on the applicable reporting rules.
- Due Date Calculations: Depend on your Admin’s setting in the Localization record’s Localized Due Date Calculation field. The following options may be configured:
- If set to Localized Case - Local Awareness Date, the Due Date is based on the Local Awareness Date and the Due in Days evaluation. If the Local Awareness Date is blank, Vault uses the New Info Date, if available, or the Receipt Date.
- If set to Global Case - New Info Date, the Due Date is based on the New Info Date and the Due in Days evaluation. If the New Info Date is blank, Vault uses the Receipt Date.
In addition, Vault rolls up the Due Date and Due in Days values to the related Case Product Registration fields.
Vault does not generate Transmissions through these actions.
Expectedness
On Domestic Cases for Japan, Expectedness records are not generated for Case Products with registrations that are reportable to the PMDA. Instead, Expectedness evaluations appear on Localized Case Assessment records. At the Case-level, Expectedness is set using the most reportable evaluation across all non-PMDA Expectedness records and Localized Case Assessments from the Japan Localized Case.
When the Localized Case Assessment Expectedness or Expected (status) value is overridden, the update is synced to the Global Case for Domestic Cases, but not for Localized Cases.
Note: Non-Vault Owner users cannot update the Expected field value on Localized Case Assessments, which would change the value of the most conservative expectedness of the Case Assessment, when the global Case is in a terminal state, such as Approved, Closed, or Superseded.
PMDA Follow-Up Information Expectedness Reevaluation
Your Admin’s configuration of the Recalculate Post-Market Expectedness on Follow-up application setting determines when Vault reevaluates the expectedness of adverse events on postmarket Cases that are reportable to the PMDA. This includes when Inbox Item information is merged into an in-flight Case or promoted to a follow-up Case.
If the setting is clear, Vault copies and reevaluates expectedness in the same way as for non-PMDA Cases.
If the setting is selected:
- On global Cases:
- For all Case Assessment Expectedness records on which the Expected (status) is Auto Calculated or blank, Vault reevaluates expectedness.
- Vault generates any new or missing Case Assessment Expectedness records for Case Products in Case Assessments.
- On domestic Localized Cases, for all Localized Case Assessments on which the Expected (status) is Auto Calculated or blank, Vault reevaluates expectedness.
Case Product Registration Deletion
When you delete a Case Product Registration, Vault deletes Localized Case Assessments and updates Localized Case Assessment Results and Local Reporting Details records that reference the deleted Case Product Registration. The following applies to Japan domestic Cases and Localized Cases.
Localized Case Assessment Result Updates
If there is only one (1) Localized Case Assessment, Vault deletes it and the associated Localized Case Assessment Result.
If the Localized Case includes multiple Localized Case Assessments (LCAs) and any reference another Case Product Registration (CPR) that shares the same Case Product (CP) as the deleted CPR, Vault updates the Localized Case Assessment Results (LCARs) for LCAs that reference the deleted CPR to reference another LCA.
For example, suppose you have:
- CP1 with
- CPR1 and LCA1
- CPR2 and LCA2
- LCAR1 with LCA1 in the Localized Case Assessment field
When Vault deletes CPR1 and LCA1, Vault updates the Localized Case Assessment field of LCAR1 to LCA2.
Local Reporting Details Updates
During Case Product Registration deletion, to update Local Reporting Details records, Vault:
- Clears the Primary Case Product Registration field of any Local Reporting Details records that reference the deleted Case Product Registration.
- Deletes any Local Reporting Details/Product Join records that reference the deleted Case Product Registration.
Case Product Deletion Through Follow-Up
If you delete a Case Product, when an Inbox Item is merged to an in-flight Case or promoted to a follow-up Case through the Inbox Item to Case Compare page, Vault:
- Deletes all associated Case Product Registrations, Localized Case Assessments, and Localized Case Assessment Results.
- Clears the Primary Case Product Registration field of any Local Reporting Details records that reference the deleted Case Product Registration.
- Deletes any PMDA Reportable Products records that reference the deleted Case Product Registration.
In Vaults configured to isolate blinded clinical trial information, Vault prevents deleting unblinded Case Products on the Inbox Item to Case Compare page. For blinded, open-label, and non-study products, Vault performs the actions listed above when Case Products are deleted.