Learn how to configure your Vault to support signal detection using EudraVigilance Data Analysis System (EVDAS) data.
About the Feature
With the 24R3 release, users can import EVDAS data into Vault in the form of electronic Reaction Monitoring Reports (eRMRs) and line listings. Users can then perform signal detection in compliance with EMA regulations.
After you have configured this feature, see the following articles for more information:
Create Custom Layouts
Create custom layouts for the following objects:
To create custom object layouts for Safety Signal:
- Navigate to Admin > Configuration > Objects > [Object] > Layouts.
- Do one (1) of the following:
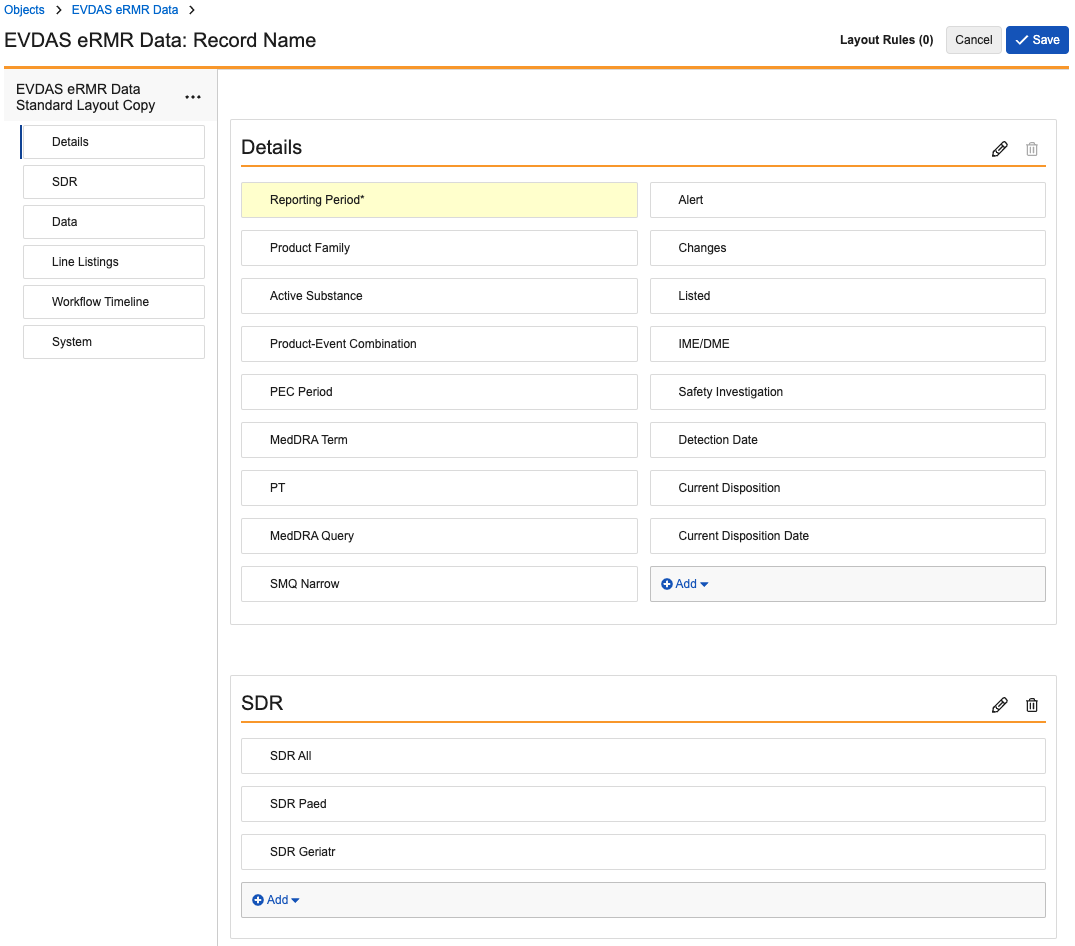
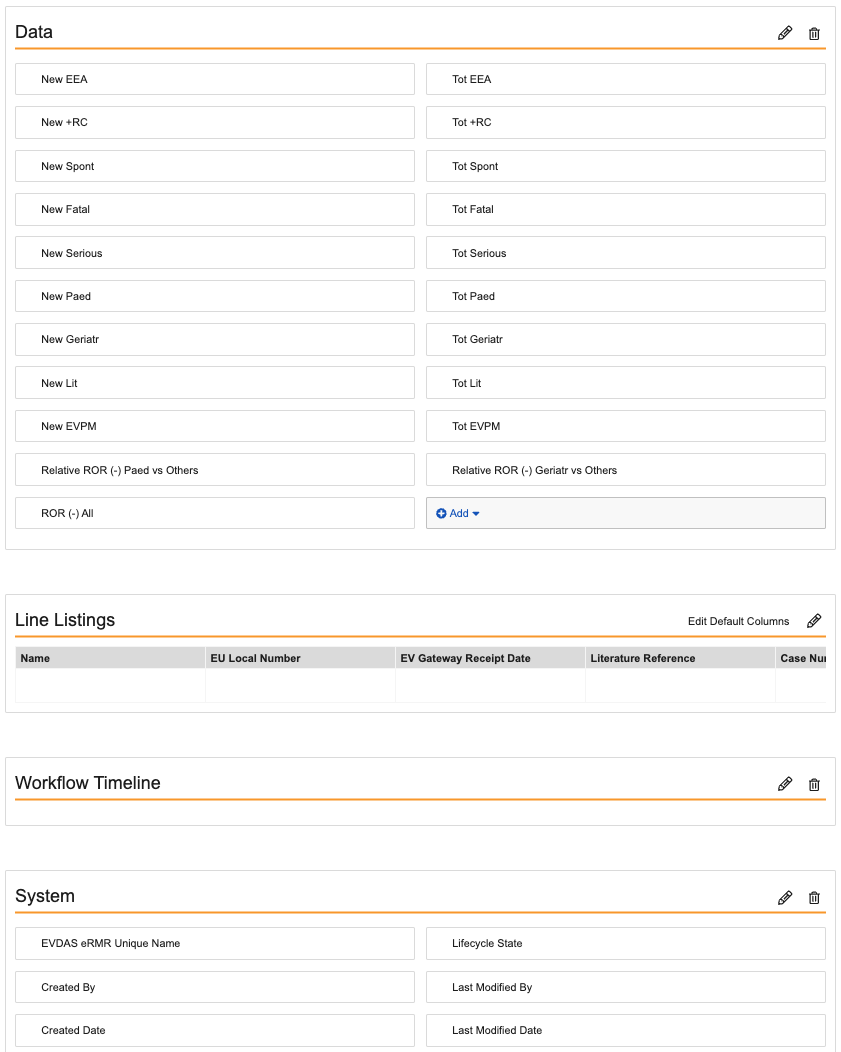
EVDAS eRMR Data
Update the EVDAS eRMR Data layout to match the following images:
Optionally, in the Line Listings section, edit the default columns to appear as follows:
- Name
- Product Family
- Reporting Period
- EU Local Number
- Worldwide Unique Case Identification
- Case Number
- Case Version
- EV Gateway Receipt Date
- Report Type
- Literature Reference
- Parent Child Report
- Patient Age Group
- Patient Age Group (as per reporter)
- Patient Sex
- Primary Source Qualification
- Primary Source Country for Reg. Purposes
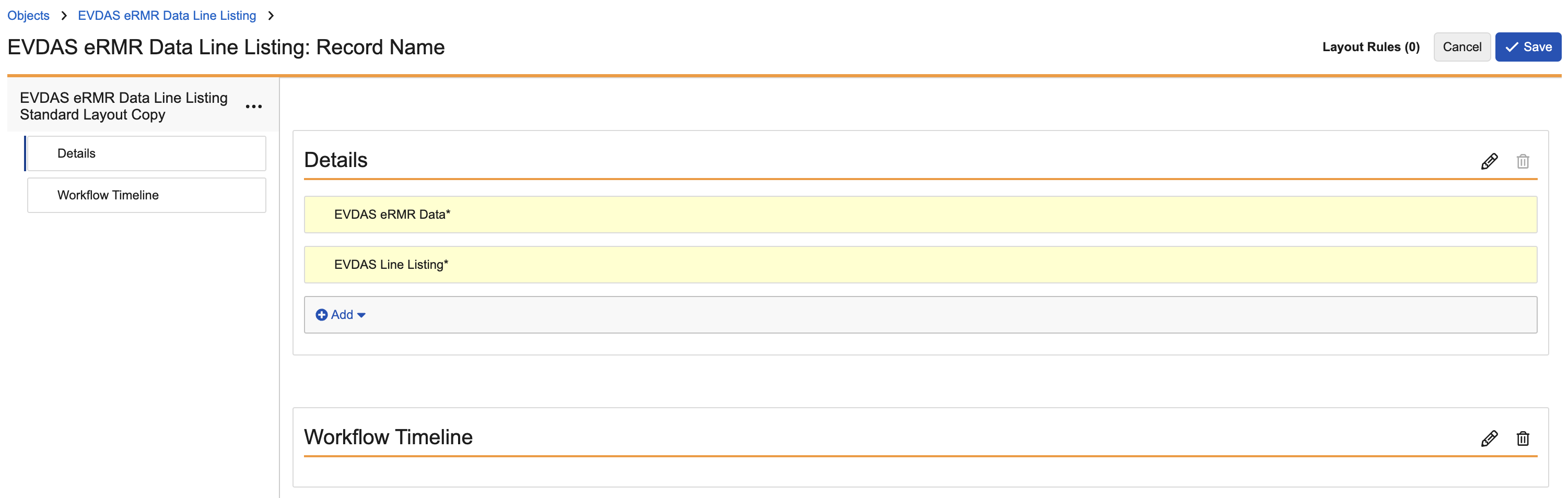
EVDAS eRMR Data Line Listing
Update the EVDAS eRMR Data Line Listing layout to match the following image:
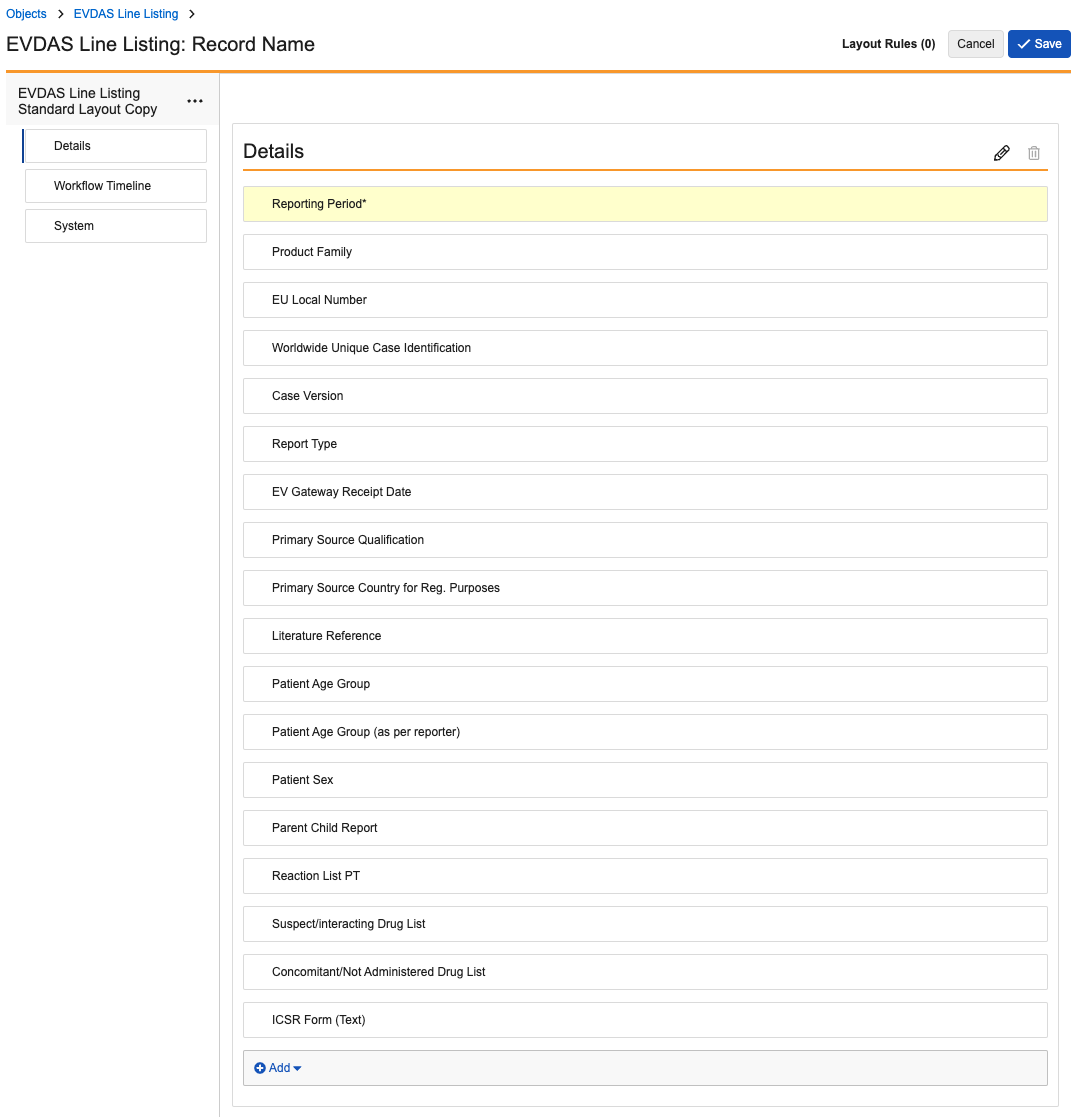
EVDAS Line Listing
Update the EVDAS Line Listing layout to match the following image:
Update the EVDAS eRMR Data Object
To update the EVDAS eRMR Data object, navigate to Admin > Configuration > Objects > EVDAS eRMR Data and perform the following configuration:
Update Fields
Update the Active Substance and PT fields to display in default lists and hovercards:
- Select the Fields tab.
- Select Active Substance.
- Select Edit.
- Select the Display in default lists and hovercards checkbox.
- Select Save.
- Repeat the above steps for the PT field.
Update the List Layout
To update the EVDAS eRMR Data list layout:
- Navigate to the List Layout tab and select Reorder.
- Drag field names so the list layout appears in the following order:
- EVDAS eRMR Unique Name
- Product Family
- MedDRA Term
- MedDRA Query
- Active Substance
- PT
- Alert
- Changes
- Listed
- IME/DME
- SDR All
- SDR Geriatr
- SDR Paed
- New EEA
- Tot EEA
- New EVPM
- Tot EVPM
- New Fatal
- Tot Fatal
- New Geriatr
- Tot Geriatr
- New Paed
- Tot Paed
- New +RC
- Tot +RC
- New Serious
- Tot Serious
- New Spont
- Tot Spont
- ROR (-) All
- Reporting Period
- Current Disposition
- Safety Investigation
- Detection Date
- Select Save.
Insert the EVDAS eRMR Section to Object Layouts
Insert the EVDAS eRMR section to the following object layouts:
- Safety Investigation object > Safety Investigation Detail Page Layout
- Signal Reporting Period object > Signal Reporting Period Detail Page Layout
To do this:
- Navigate to Admin > Configuration > Objects > [Object] > [Layout].
- After the Statistical Data section, insert the EVDAS eRMRs related object section.
- In the Add Related Object Section window, complete the following information:
- Related Object: From the drop-down, select EVDAS eRMR Data.
- Section Name: Enter
EVDAS eRMRs. - Creation Option: Select Create record in pop-up dialog.
- Select Done.
- Select Save.
Grant Object Permissions
Ensure your permission sets are updated to grant the appropriate level of access for users to objects and fields according to your organization’s process. Manage object permissions from Admin > Users & Groups > Permission Sets > [Permission Set] > Objects > [Object].
The following table summarizes the recommended level of access for your permission sets:
| Object | Permissions |
|---|---|
| EVDAS eRMR Data | Read, Create, Edit, and Delete access |
| EVDAS eRMR Data Line Listing | Read, Create, Edit, and Delete access |
| EVDAS Line Listing | Read, Create, Edit, and Delete access |