The tabs that appear in the navigation bar depend on your account permissions and your Vault’s custom configuration.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
For information on navigation items in all Safety Suite Vaults, see Navigate Safety Suite.
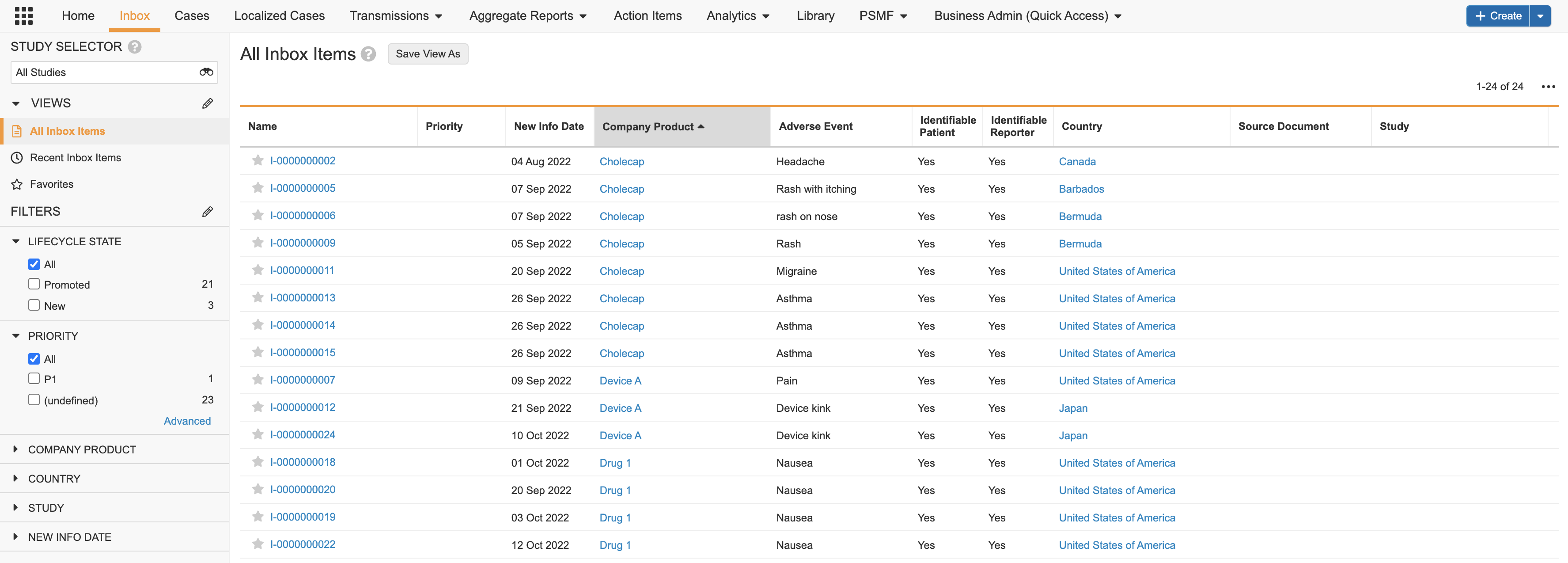
Inbox
Perform intake on the Inbox tab.
The Inbox organizes Inbox Items into a detailed table, providing at-a-glance key information. You can sort Inbox Items in ascending or descending order using any column in the table, such as New Info Date, Product, Organization, Seriousness, Due Date, and more.
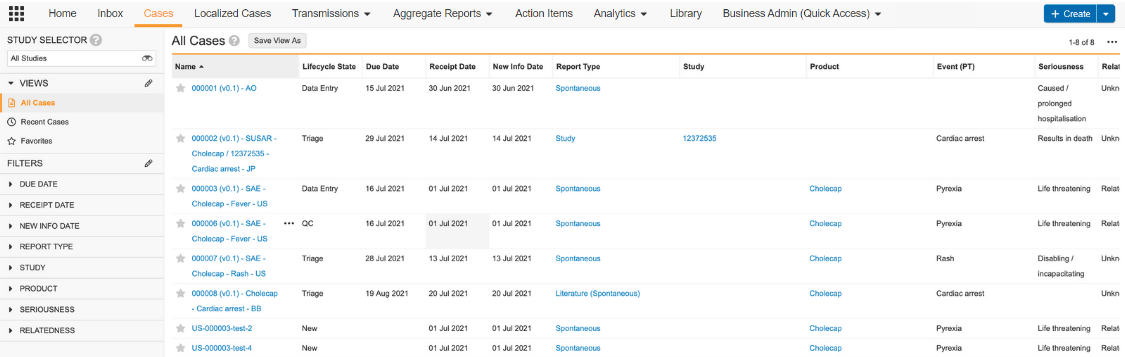
Cases
The Cases tab contains all Safety Cases. To create a Case, you must create an Inbox Item from the Inbox tab and then promote the Inbox Item to a Case. You cannot create a Case directly from the Cases tab.
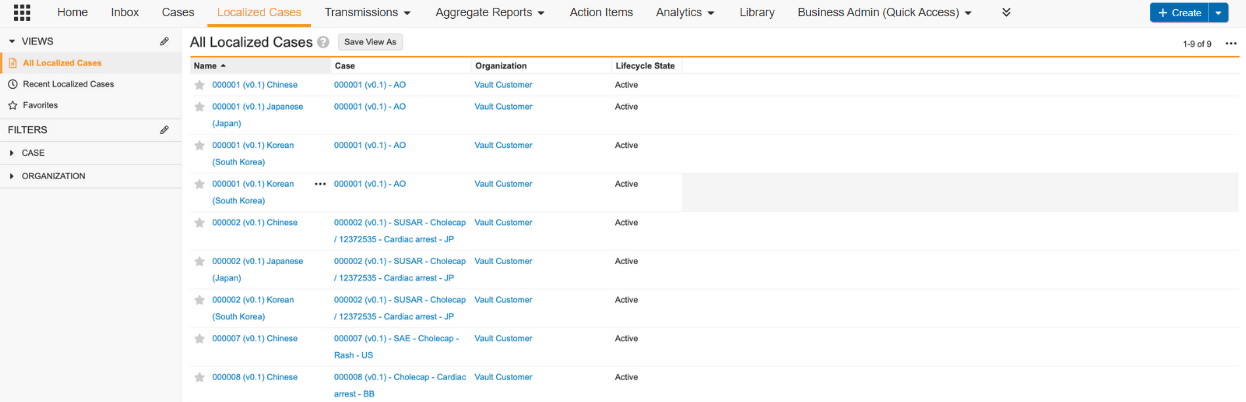
Localized Cases
Certain Vaults may contain the Localized Cases tab for quick access to Localized Cases.
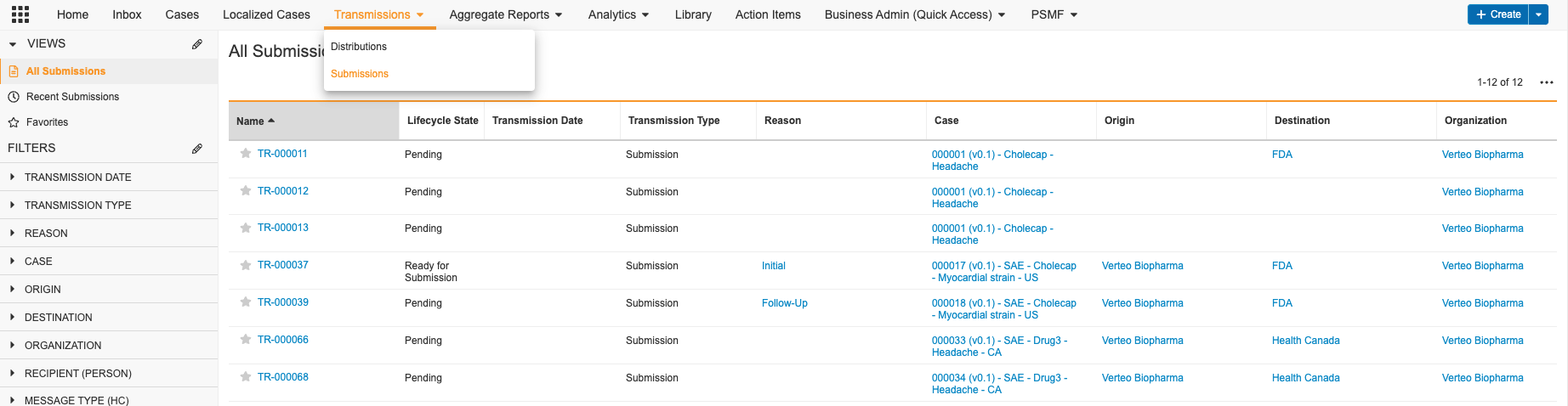
Transmissions
Safety supports both manual and automated submissions of Individual Case Safety Reports (ICSRs) to the FDA. Manage Submissions from the Transmissions tab.
If you do not see the Transmissions tab, you may not have permission to participate in Submission workflows. By default, only users assigned the Head of Safety and Distribution Manager roles can participate in Submissions.
Case Collections
The Case Collections tab includes groups of Case Numbers collected for the purpose of non-submission reporting, for example, reporting to an auditor or ethics committee about a specific subset of Cases. Case Numbers can be added or removed from Case Collections. Safety Report Document Collections can be generated from Case Collections, so you can export multiple ICH E2B(R3) or CIOMS I forms at once. If safety reports have been generated, a link to the associated binder appears on the Case Collection. This facilitates easy downloading and sharing of safety reports with partners or relevant health authorities.