Vault supports Case processing for Domestic Cases. Domestic Case processing provides region-specific fields and dual-language fields for entering data in a non-English language on a Case.
A Domestic Case is for cases that must be reported to the same jurisdiction where the adverse event occurred, in the local, non-English language. If a product involved in a Domestic Case has a registration to an English-reporting country (such as the FDA and EMA), you can enter English translations on the Domestic Case.
Using a Domestic Case, you can perform data entry in the local language the report was received in, while translating to English. This is a different workflow from localizing a Global (English) Case to a different language, which is through Localized Cases.
Domestic Cases support region-specific fields for the following agencies:
- PMDA (Japan)
- NMPA (China)
- MFDS (Korea)
For information on region-specific fields for reporting to the PMDA, see Complete Intake and Process Cases for the PMDA.
Prerequisites
For Vaults created earlier than 21R3, Admins must Enable Domestic Case Processing to process Domestic Cases.
For Vaults created earlier than 21R2, Admins must complete the following configuration:
- Enable Localized Submissions and Translations Support
- Enable Local Fields for NMPA (China) for Chinese case processing
- Enable Local Fields for MFDS (Korea) for Korean case processing
To include Case Products with the Drug Role of Drug Not Administered when generating Assessments, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered. Otherwise, Assessment generation considers only Case Products with the Suspect or Interacting Drug Role.
Create a Domestic Case
Vault generates a Domestic Case after you promote an Inbox Item with the Localization field set to a non-English value. See Perform Local Language Intake for more information.
You can also create a Follow-up Domestic Case from a Global type Inbox Item or merge a Global type Inbox Item to an In-flight Domestic Case. See Perform Inbox Item to Case Compare for more information.
When the Auto-select Inbox Item Localization by Reporter Country feature is enabled, for primary Reporter-type Contacts, if the Country has a Localization record, the Localization field on the Inbox Item is automatically set based on the Reporter’s Locale. See the Country field on the Inbox Item Field Reference for more information.
Your Admin can also configure Vault to set Inbox Item Localization using both the Primary Reporter Country and Report Type. See the logic for how Vault auto-sets Localization.
After promoting an Inbox Item (created through Intake in a non-English language) to a Domestic Case, you can view and process Domestic Cases entirely in the reported language from the Data Entry through to the Medical Review stage of the Case processing lifecycle. Domestic Case Processing is completed on the Cases tab.
Regenerate a Domestic Case
If a Domestic Case did not successfully generate after promoting an Inbox Item, you can use the Re-generate Domestic Case action to trigger Domestic Case generation. This action is available from the All Actions menu on a Localized Case.
The Re-generate Domestic Case action generates all child records or any child records that have been deleted. For example, if the Domestic Case includes Parent Information, Vault generates both a Domestic Localized Case and a Domestic Localized Parent Information Case.
Set Vault Default Language
You can view fields in Vault in your preferred language by selecting a language from your User Profile. When you change this setting, all field labels and picklist values in Vault will display in the selected language. The Default Localization field determines the language in Object Reference fields, such as Report Type.
Note: If the Default Localization field is blank, Vault automatically refers to the Language field to determine the language for the picklist field values.
Enter Translations
When an Inbox Item is created through Intake in a non-English language with the Localization field set to a non-global value, you can enter Inbox Item data in the reported language for all sections of the Inbox Item.
See Create an Inbox Item Manually for more information on creating inbox items.
Localization Scope
When processing domestic Cases, Vault includes dual-entry fields so you can perform data entry in both the reporter’s local language and English at the same time. By default, text, picklist, and object fields are available for dual-language entry. However, your Admin can configure Localization Scope to limit dual-language entry to Narratives, Company Comments, or both.
E2B file export respects the Localization Scope setting. For example, if the Localization Scope is Narrative, only the localized narrative content is exported on E2B files. All other fields are English only.
Vault also considers Localization Scope before generating Parent Information Cases for domestic Cases. If the Localization Scope is Narratives, Company Comments, or both, Vault does not generate domestic Parent Information Cases.
Manual Text Translation
You can manually translate text fields during intake. For each text field that supports dual language intake, there are two fields:
- In the first field, enter the information in the language the adverse event was reported in.
- In the English (
 ) field, enter the English translation.
) field, enter the English translation.
Code Multilingual MedDRA Terms
You can enter MedDRA terms in the reported language of the Case. For more information about how to code multilingual MedDRA terms, see Code MedDRA Terms.
Domestic State Code Mapping
For domestic Cases originating in a country, such as Spain or Italy, where the state code is required in submissions to a specific agency, such as the EMA, Vault provides a configurable Country State/Province library with Domestic State Codes. If configured by your Admin, upon initial or follow-up domestic Case creation, Vault selects the Use Domestic State Code checkbox on the primary Reporter-type Localized Case Contact. This includes when a follow-up domestic Inbox Item is merged into or promoted to a follow-up for an initial global Case. When the Use Domestic State Code checkbox is selected, during E2B(R3) import and export, Vault attempts to map the State/Province value on the Case Contact to a Country State/Province record.
On import, Vault selects the Use Domestic State Code checkbox on the primary Reporter-type Localized Case Contact and clears the value in the State/Province field.
On export, Vault populates the C.2.r.2.5 Reporter’s State or Province data element on EMA E2B(R3) files as follows:
- For countries within the jurisdiction of the EMA:
- For primary Reporter-type Case Contacts on global Cases and primary Reporter-type Localized Case Contacts on Localized Cases with the Use Domestic State Code checkbox selected, Vault maps the State/Province to a Country State/Province record. If the record includes a Domestic State Code, Vault exports the code. Otherwise, Vault exports the Name of the Country State/Province record.
- For non-primary Reporter-type Case Contacts on global Cases and non-primary Reporter-type Localized Case Contacts on Localized Cases with the Use Domestic State Code checkbox selected, Vault exports the Name of the Country State/Province record.
- For countries outside the jurisdiction of the EMA:
- For all primary and non-primary Reporter-type Case Contacts on global Cases and Reporter-type Localized Case Contacts on Localized Cases with the Use Domestic State Code checkbox selected, Vault exports the Name of the Country State/Province record.
In all of the above scenarios, if a Country State/Province record is not available or the Use Domestic State Code checkbox on the Localized Case Contact is clear, Vault exports the State/Province value on the Case Contact or Localized Case Contact.
Text Field Mapping on Case Promotion
When a Domestic Case is created upon Case promotion, the Inbox Item local text fields (that have data entered in their native language) are mapped to the corresponding local text fields on the Domestic Case in the same language.
The following table describes how Vault maps Inbox Item local text fields to the local text fields on the Domestic Case:
| Inbox Item | Domestic Case | ||
|---|---|---|---|
| Section | Field | Object | Field |
| Details > Awareness Details | Reporter's Comments (Native) | Localized Case | Reporter's Comments |
| Details > Awareness Details | Reporter's Comments (English) | Case Version | Reporter's Comments |
| Case Contacts > Contact | Additional Information (Native) | Localized Case Contact | Additional Information |
| Case Contacts > Contact | Additional Information (English) | Case Contact | Additional Information |
| Case Contacts > Contact | Organization (Native) | Localized Case Contact | Organization |
| Case Contacts > Contact | Organization (English) | Case Contact | Organization |
| Case Contacts > Contact | Department (Native) | Localized Case Contact | Department |
| Case Contacts > Contact | Department (English) | Case Contact | Department |
| Case Contacts > Primary Reporter | State/Province | Case Contact > Reporter | State/Province |
| Product | Product (Native) | Localized Case Product | Product (Reported) |
| Product | Product (English) | Case Product | Product (Reported) |
| Product | Indication (Native) | Localized Case Product Indication | Name (Reported) |
| Product | Indication (English) | Case Product Indication | Name (Reported) |
| Product > Case Product > Dosage | Dose Text (Native) | Localized Case Product Dosage | Dose Text |
| Product > Case Product > Dosage | Dose Text (English) | Case Product Dosage | Dose Text |
| Medical Events > Medical history & Concurrent Conditions | Event (Native) | Localized Case Medical History | Name (Reported) |
| Medical Events > Medical history & Concurrent Conditions | Event (English) | Case Medical History | Name (Reported) |
English Field Translation Mapping
On Case promotion, only the  field translations are mapped to the Global Case. The field values in the local language are not copied over to the Global Case.
field translations are mapped to the Global Case. The field values in the local language are not copied over to the Global Case.
The following table describes how Vault maps text fields with local and English values on Case promotion:
| Inbox Item | Case | ||
|---|---|---|---|
| Section |  Field Field |
Object | Field |
| Details | Reporter Comments | Case (Narrative) | Reporters Comments |
| Case Contact | Additional Information | Case Contact | Additional Information |
| Case Contact | Organization | Case Contact | Organization |
| Case Contact | Department | Case Contact | Department |
| Product | Product (Reported) | Case Product | Product (Reported) |
| Product > Dosage | Dose Form (Custom)* | Case Product Dosage | Dose Form |
| Product > Dosage | Patient RoA (Custom)* | Case Product Dosage | Patient RoA |
| Product > Dosage | Dose Text | Case Product Dosage | Dose Text |
| *Text translations are only available when entering custom Dose Forms or Patient RoAs. Translations for standard system-provided Dose Forms and Patient RoA values are automatically mapped upon Case promotion. | |||
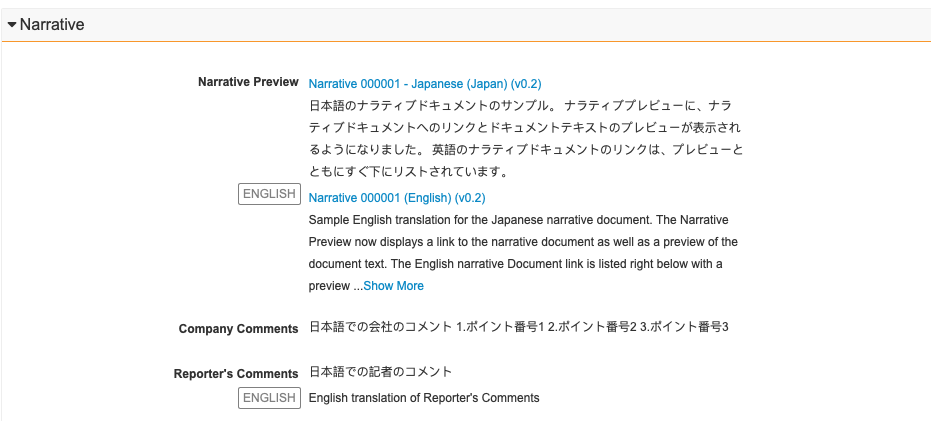
About the Local Narrative Preview
When you promote an Inbox Item to a domestic Case, Vault generates a localized and English narrative document based on your Admin’s configuration, which you can review and access in the Narrative section. You can edit the generated narrative document or upload another document to replace the generated narrative. For more information, see Generate a Case Narrative.
Note: You cannot edit the narrative previews in this section.
PMDA-Specific Sections
The following related sections are available only for processing Cases that may be reportable to the PMDA:
- Case Product Registrations
- Local Reporting Details
- PMDA Reportable Products
- Case Comments
For information on these sections, see Complete Intake and Process Cases for the PMDA.