Veeva Safety supports Standardised MedDRA Queries (SMQs), custom MedDRA Queries (CMQs), and MedDRA Query Building Blocks, allowing you to use queries to find patterns in previously processed Cases and discover new adverse events. The following table describes the MedDRA queries available in your Vault:
| Query Type | Description |
|---|---|
| Standardised MedDRA Query (SMQ) | Standardised MedDRA Queries (SMQs) are defined by MedDRA and can be imported into your Vault, but cannot be modified. Each SMQ is a group of MedDRA Preferred Terms (PTs) for a medical condition or area of interest. To view the SMQs loaded in your Vault, navigate to Business Admin > Objects > MedDRA Queries. SMQs are labeled with |
| Custom MedDRA Query (CMQ) | Custom MedDRA Queries (CMQs) are defined by your organization. CMQs can include Sub-Queries, which support creating a parent-child relationship between different CMQs. |
| MedDRA Query Building Block |
MedDRA Query Building Blocks are defined by your organization. MedDRA Query Building Blocks can combine SMQs, CMQs, and MedDRA terms at any level of the hierarchy. They can be applied to multiple Datasheets and Watchlists that have the same list of expected and unexpected adverse events. Building Blocks differ from CMQs in that they can reference other MedDRA Queries to create a single query. This supports easier maintenance of Datasheets and Watchlists. When a MedDRA Query Building Block is updated, you can apply the changes to all Datasheets and Watchlists that reference it using a single action. |
Learn more about running a MedDRA Query to search for Cases.
Prerequisites
- You must be a Business Admin to manage MedDRA Queries.
- Ensure that your Vault has the configuration required to support the following features, as needed:
Import Standardised MedDRA Queries
Vault updates SMQs when the Upgrade to Latest Version action runs on the MedDRA Dictionary. You can manually import earlier versions of SMQs, provided the MedDRA ZIP file version matches your Vault’s current active MedDRA version:
- Navigate to Business Admin > Objects > Dictionaries.
- On the MedDRA Dictionary page, expand the Attachments section, then select Upload.
- Browse to the MedDRA ZIP file, select the file, then select Open.
- In the Details section, enter the license key for the MedDRA ZIP file.
- Select Save.
- From the All Actions menu, select Import Standard MedDRA Queries.
Note: Vault supports importing only English SMQs.
Vault imports the SMQs and their associated terms. In Vaults deployed before the 24R1 release, imported SMQs referenced MedDRA Lowest Level Terms (LLTs). Importing in 24R1 and later updates SMQs to reference MedDRA Preferred Terms (PTs).
Run a MedDRA Query
Safety includes a number of pre-configured MedDRA SMQ/CMQ multi-pass reports. Reports can contain a Root Query (only for a query with multiple subqueries) or Multiple Queries (only for searching across queries with no nested subqueries). Run these reports to search for Cases that match one or more SMQs/CMQs.
- SMQ/CMQ Search by Study Using Root Query Report: Search for Cases under a certain Study and with an Adverse Event that matches a given root query’s hierarchy.
- SMQ/CMQ Search by Study Across Multiple Queries Report: Search for Cases under a certain Study and with an Adverse Event that matches one or more given queries.
- SMQ/CMQ Search by Product Using Root Query Report: Search for Cases with a certain Product and an Adverse Event that matches a given root query’s hierarchy.
- SMQ/CMQ Search by Product Across Multiple Queries Report: Search for Cases with a certain Product and an Adverse Event that matches one or more given queries.
You can run these reports as-needed, schedule flash reports, or set up dashboards to monitor SMQs and CMQs. Manage Reports provides more information on using reports.
Configure MedDRA Queries
You can configure custom MedDRA Queries (CMQs) and MedDRA Query Building Blocks to meet your organization’s adverse event monitoring needs. Custom MedDRA Queries can be set up as non-hierarchical or hierarchical, as follows:
- Non-hierarchical MedDRA Queries:
- MedDRA Terms can be added at any level of the MedDRA hierarchy
- MedDRA Query Building Blocks can be defined, including multiple SMQs and CMQs
- Hierarchical MedDRA Queries:
- Sub-Queries can be added, which support creating a parent-child relationship between different CMQs
Add a Custom MedDRA Query
To add a custom MedDRA Query:
- Navigate to Business Admin > Objects > MedDRA Queries.
- Select Create.
- On the Create MedDRA Query page, complete the following fields:
- Organization: Select the organization that should have access to the query.
- Name: Enter a name to label the query.
- Description: Enter a description.
- Hierarchical: To create a hierarchical CMQ that contains Sub-Queries, select Yes. To create a regular Medical Query that includes a combination of MedDRA Terms and Building Blocks, select No. If you create a hierarchical CMQ, you cannot add MedDRA Terms to the parent CMQ. You can add the MedDRA Terms to the Sub-Queries.
- Submission Rule Scope: If using MedRA query parameters in a reporting rule, select one or both scopes for the MedDRA Query:
- Select Broad to query MedDRA Terms where the Scope field is set to Broad.
- Select Narrow to query MedDRA Terms where the Scope field is set to Narrow. To learn more, see Reporting Rule Parameter Reference.
- Select Save.
After adding a custom MedDRA Query, add MedDRA Terms and MedDRA Query Building Blocks or Sub-Queries, as required.
Add a Sub-Query
You can add Sub-Queries to hierarchical MedDRA Queries:
- On a hierarchical MedDRA Query page, expand Sub-Queries, and then select Create.
- Complete the Create MedDRA Query window, and then select Save.
- Add additional Sub-Queries, as required.
Add a MedDRA Term
You can add MedDRA Terms to non-hierarchical MedDRA Queries and Sub-Queries:
- On a non-hierarchical MedDRA Query or Sub-Query page, expand MedDRA Terms, and then select Create.
- In the Create MedDRA Criteria window, complete the applicable fields.
- Add additional MedDRA Terms, as required.
MedDRA Criteria Fields
| Field | Description |
|---|---|
| MedDRA Term | Enter the reported term in the text field to code the MedDRA term |
| Scope | Select whether the terms in the MedDRA Query record are narrow, broad, or both. Select Narrow or Broad to pull in values with the corresponding scope applied on the SMQ or CMQ term. Leave blank to pull in all values. |
Add a MedDRA Query Building Block
You can add MedDRA Query Building Blocks to non-hierarchical MedDRA Queries:
- On a non-hierarchical MedDRA Query or Sub-Query page, expand MedDRA Query Building Blocks and then select Create.
- In the MedDRA Query field, select an SMQ or CMQ and then select Save. You can also select Save + Create to immediately add another SMQ or CMQ to your Building Block.
- Add additional Building Blocks, as required.
Apply MedDRA Queries to Datasheets and Watchlists
When you create a MedDRA Query, you can apply it to Datasheets and Watchlists when managing individual Datasheets or Watchlists. You can also add MedDRA Queries to Datasheets and Watchlists from within MedDRA Queries.
Add MedDRA Queries to a Datasheet
To add a MedDRA Query to a Datasheet:
- Select a MedDRA Query and expand the Datasheet MedDRA Queries section.
- Select Create.
- Complete the applicable fields.
- Select Save.
Datasheet MedDRA Queries Fields
| Field | Description |
|---|---|
| Datasheet | Select a Datasheet from the dropdown list. |
| Scope | Select whether the terms in the MedDRA Query record are Narrow, Broad, or both. Select Narrow or Broad to pull in values with the corresponding scope applied on the SMQ or CMQ term. Leave blank to pull in all values. |
| Expectedness | Select whether all the terms in the MedDRA Query are Expected or Unexpected. |
| Seriousness Exclusion | To define conditions for which the listed events are always Unexpected, select one or more seriousness criteria. When this field is defined, if Vault Safety evaluates Expectedness for a Case Adverse Event with any of the MedDRA Query record terms and one or more matching Seriousness values, the system populates Expectedness as "No" (Unexpected). If you populate this field, the system ignores the Unexpected Seriousness Criteria setting on the Datasheet for these terms. |
| Active Date Start | To specify when the terms were approved to be listed on the Datasheet, enter the date of approval. The date is inclusive. This field applies only to Clinical Trial Study Cases. This setting impacts expectedness evaluation in aggregate reports (DSUR, PBRER, and PSUR). Active Range for Expectedness in Aggregate Reports provides more information. |
| Active Date End | Optionally, to specify the last day when the terms were approved to be listed on the Datasheet, enter the end date. The date is inclusive. This field applies only to Clinical Trial Study Cases. The day after the Active Date End is the first day the terms are considered unlisted and unexpected. If you don't specify an end date, the term is considered actively approved and expected. This setting impacts expectedness evaluation in aggregate reports (DSUR, PBRER, and PSUR). Active Range for Expectedness in Aggregate Reports provides more information. |
| Description | Enter a description of the expected adverse events. |
| Age Range Start | Specify the earliest patient age for which a term is expected or unexpected. Enter a number and unit. The date is inclusive. Specifying an Age Range Start, but not an Age Range End, means the term applies to patients of a minimum age. |
| Age Range End | Specify the latest patient age for which a term is expected or unexpected. Enter a number and unit. The date is inclusive. Specifying an Age Range End, but not an Age Range Start, means the term applies to patients up to a maximum age. |
| Sex | Specify the sex for which a term is expected or unexpected. Select an option from the picklist or leave blank to include all sexes. |
Add MedDRA Queries to a Watchlist
To add a MedDRA Query to a Watchlist:
- Select the MedDRA Query and expand the Watchlist MedDRA Queries section.
- Select Create, then complete the following fields:
- Watchlist: Select a Watchlist from the dropdown list.
- Scope: Select whether the terms in the MedDRA Query are narrow, broad, or both. Select Narrow or Broad to pull in values with the corresponding scope applied on the SMQ or CMQ term. Leave blank to pull in all values.
- Select Save.
Updating Datasheet and Watchlist Terms
Adding or changing a MedDRA Query does not apply the terms to linked Datasheets and Watchlists automatically. When you are ready to make the changes available for use during Case processing, go to the All Actions menu and then select Update Datasheet and Watchlist Terms. The changes are then applied and available for use by Case Processors.
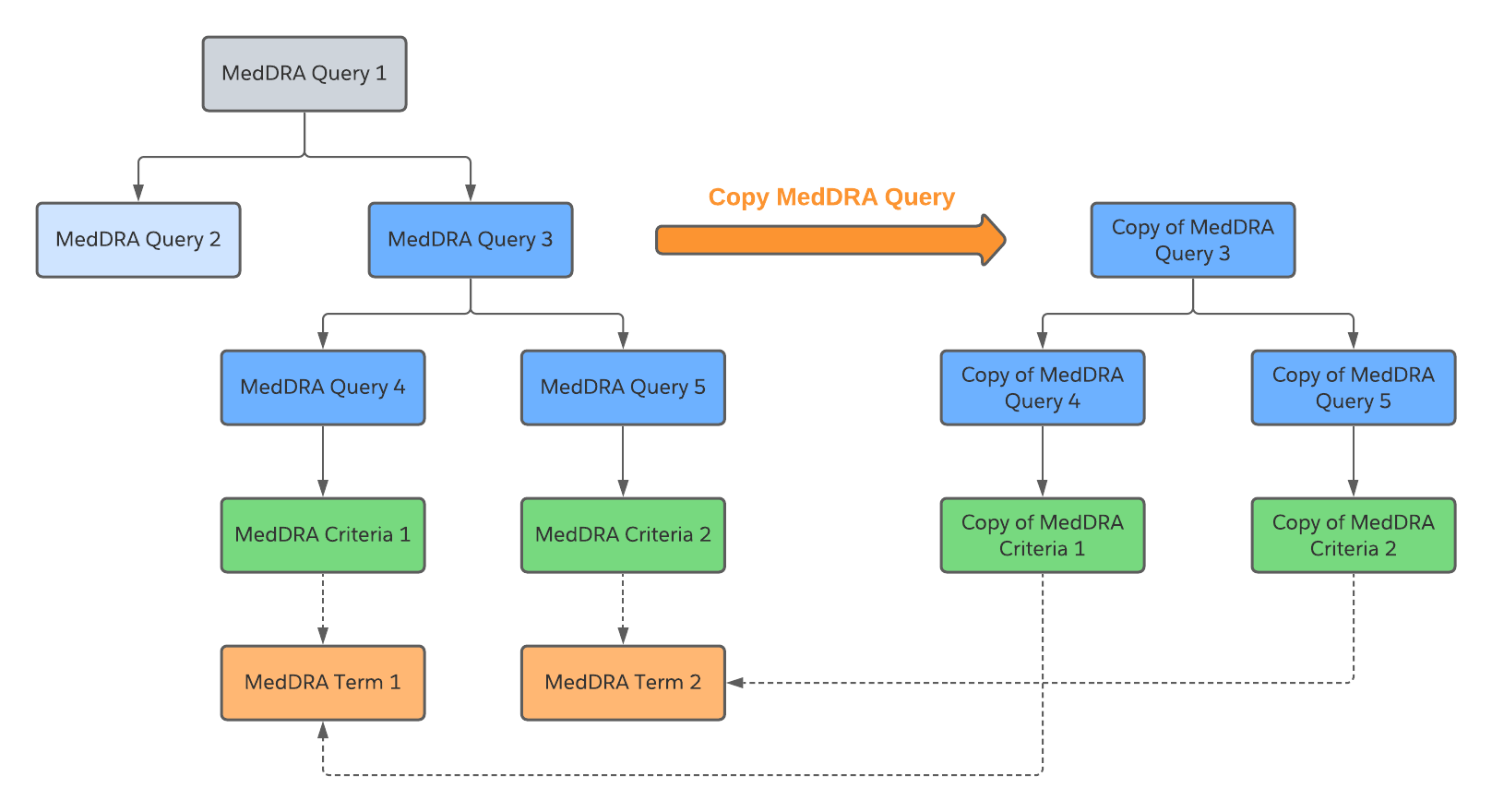
Copy a MedDRA Query
To perform a deep copy of a MedDRA Query to create a duplicate of the MedDRA Query and all its associated records, such as the MedDRA Criteria:
- Navigate to Business Admin > Objects > MedDRA Queries.
- Select Actions beside a MedDRA Query.
- Select Copy MedDRA Query.
Vault copies the MedDRA Query and its associated MedDRA Criteria. Both the copy and original query are linked to the respective MedDRA Term.
Note: When you copy a MedDRA Query with MedDRA Query Building Blocks, the MedDRA Query do not appear on the new MedDRA Query. However, all of the MedDRA Terms within the Building Blocks appear in the MedDRA Terms section of the new MedDRA Query. This is a known limitation that will be addressed in a future release.
The following diagram shows the MedDRA Query deep copy process:
The Copy of MedDRA Query 3 does not have a Parent or Root query but it is the Parent/Root query for the Copy of MedDRA Query 4 and 5.