In addition to the general reporting rules engine, Veeva Safety supports evaluating rules for cross reporting scenarios, such as FDA IND-to-NDA and cross-trial reporting. The following list describes the difference between general and cross reporting:
- In general reporting, Vault evaluates either Study Registrations or Product Registrations depending on the report type and study type of the Case. In other words, Vault generates either investigational or marketing reports, not both.
- For cross reporting, Vault evaluates additional investigational or marketing registrations, based on where the Company Products on the Case (or their Substances) are marketed or investigated worldwide. Cross reporting obligations are evaluated after Submissions are generated for general reporting.
For both general and cross reporting, Vault evaluates only active Product Registrations and Study Registrations when determining the reportability of a Case.
Safety supports the following scenarios to automatically generate additional Submissions for cross reporting:
| Scenario | Description |
|---|---|
| Investigational to Marketing | After evaluating a clinical trial Case for general reporting based on its Study Registrations, Vault evaluates whether any company Case Products or their Substances are marketed elsewhere. This scenario is supported for both same-agency (for example, FDA IND-to-NDA) and cross-agency reporting. |
| Investigational to Investigational | After evaluating a clinical trial Case for general reporting based on its Study Registrations, Vault evaluates whether any company Case Products or their Substances are investigated in other studies. |
| Marketing to Marketing | After evaluating a postmarket Case for general reporting based on its Product Registrations, Vault evaluates whether any company Case Products or their Substances are marketed elsewhere. |
| Marketing to Investigational | After evaluating a postmarket Case for general reporting based on its Product Registrations, Vault evaluates whether the company Case Products or their Substances are investigated elsewhere. |
Note: When generating Submissions for an unknown formulation Product, Vault does not evaluate cross reporting obligations for other Products within its Product Family, preventing duplicate report generation.
The Reporting Scenario parameter on a Safety Rule controls whether one or more cross reporting scenarios apply to a rule. Certain rules in the FDA Reporting Rule Set support cross reporting. Cross reporting scenarios can be added to additional rules using the Reporting Scenario parameter. Contact Veeva Services for assistance in adding custom cross reporting rules.
During cross reporting evaluations, Vault uses the registration category to match the relevant cross reporting scenarios. Product Registrations and Study Registrations can be categorized as Investigational or Marketing.
Generated Submissions must have a unique mapping to a Transmission Profile. In other words, Vault does not generate multiple Submissions using the same Transmission Profile.
On a Vault-generated Submission, the Transmission Message attachment contains a SafetyRuleLog audit log for the reporting rules and scenarios evaluated.
Cross Reporting for Non-Company Sponsored Products and Studies
Your Business Admin can specify a Product as a Non-Company Product. If the Product has one or more registrations, Vault will consider Marketing→Investigational and Marketing→Marketing (Cross-Agency) cross reporting scenarios for the Product.
In contrast, your Business Admin can specify a Study as a Non-Company Sponsored Study. Vault does not consider cross reporting scenarios for such Studies.
Cross Reporting in the FDA Reporting Rule Set
Cross reporting is supported for versions 3 and 4 of the FDA ICSR Reporting Rule Set.
The Active Rule Version on the Safety Rule Set must be set to 3 or 4 to activate the cross reporting scenarios. See Manage Reporting Rule Versions for more information.
In versions 3 and 4, the following FDA Safety Rules evaluate the Investigational to Marketing (same agency) scenario:
- Downgrade to Non-Serious
- Downgrade to SAE
- SUSAR
- SUSAR (Death)
- SUSAR (Life Threatening)
In version 4, the following FDA Safety Rules additionally evaluate the Investigational to Investigational (same agency) and Investigational to Investigational (cross agency) scenarios to support FDA E2B(R3) IND cross reporting requirements:
- Downgrade to SAE
- SUSAR
- SUSAR (Death)
- SUSAR (Life Threatening)
Enable Same Agency Cross Reporting
The Inv. to Marketing (same agency) field on an Agency determines whether Vault can generate two Submissions to the same Agency for cross reporting. This field is enabled for the FDA by default. Your Admin can enable this field on additional agencies if required.
Cross Reporting Scenarios
Each Safety Rule can specify the reporting scenarios it supports using the Reporting Scenario rule parameter.
For same-agency reporting, two Submissions can be generated for the same reporting destination (for example, FDA IND-to-NDA). For cross-agency reporting, Vault ensures only one Submission per reporting destination is generated.
Note: When generating Submissions for cross reporting, Vault ensures only one Submission per registration type (Investigational or Marketing) and Agency is generated. In the event that an Agency has both X→Investigational and X→ Marketing reporting obligations then Vault uses the Cross Report Priority field (if enabled) on the Agency to prioritize which Submissions to an Agency to generate. See Add an Organization for the options available for this field.
The following sections describe supported cross reporting scenarios.
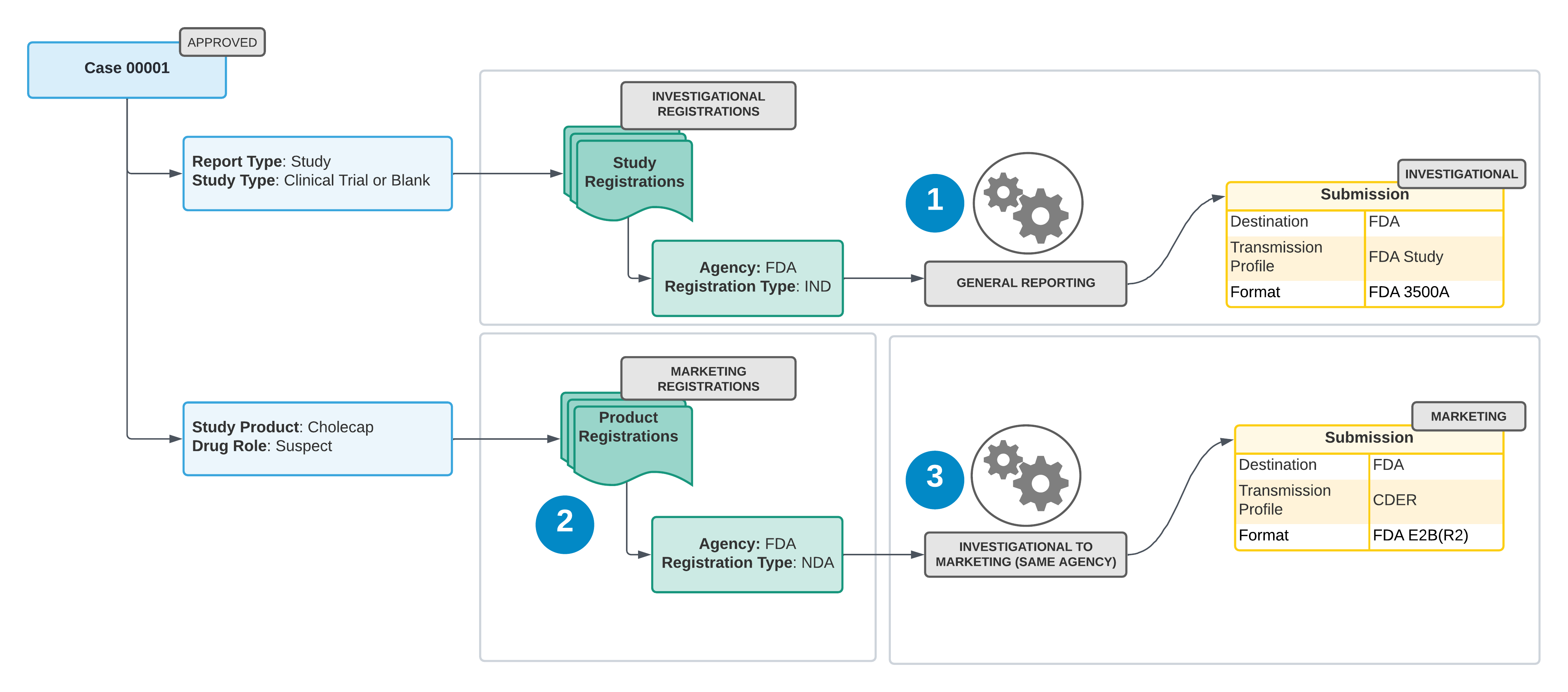
1. Investigational to Marketing (Same Agency)
This scenario applies to clinical trial Cases containing Case Products or Substances marketed in the same jurisdiction where they are being investigated.
Note: The Inv. to Marketing (same agency) setting must be enabled on the target Agency for this cross reporting scenario to execute.
-
Vault generates investigational Submissions for the applicable Agencies, based on the Study Registration countries (general reporting scenario).
- Vault queries each company Case Product with a Drug Role of Suspect or Interacting to find marketing registrations from the following sources:
- The Case Product’s Product Registrations. If the Case Product is part of a Combination Product, Vault queries the Combination Product’s Product Registrations instead. The Combination Product Registration designated as the PMOA takes precedence above other registrations.
- The Product Registrations of any Company Product that shares the same Product Substance (active ingredient) with the Case Product.
- Vault generates marketing Submissions for Product Registrations that share the same reporting destination as the Investigational Submissions generated by the general reporting scenario. To generate these Submissions, a matching Transmission Profile must exist.
Note: When evaluating cross reportable registrations, only shared products or product substances are considered as eligible products.
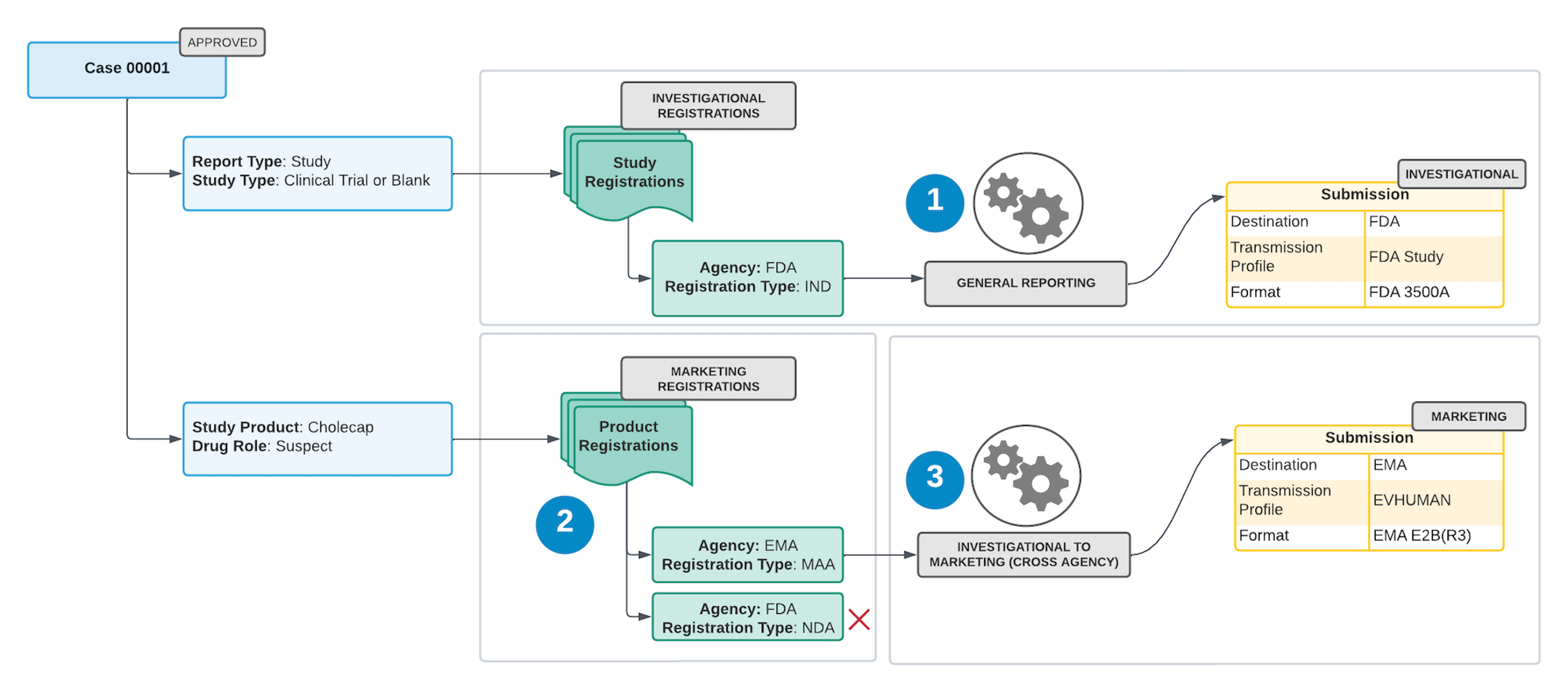
2. Investigational to Marketing (Cross Agency)
This scenario applies to clinical trial Cases containing Case Products or Substances marketed in a different jurisdiction than where they are being investigated.
-
Vault generates investigational Submissions for the applicable agencies, based on the Study Registration countries (general reporting scenario).
- Vault queries each company Case Product with a Drug Role of Suspect or Interacting to find marketing registrations from the following sources:
- The Case Product’s Product Registrations. If the Case Product is part of a Combination Product, Vault queries the Combination Product’s Registrations instead. The Combination Product Registration designated as the PMOA takes precedence above other registrations.
- The Product Registrations of any Company Product that shares the same Product Substance (active ingredient) with the Case Product.
- Vault generates marketing Submissions for Product Registrations with reporting destinations that are different from the Investigational Submissions generated by the general reporting scenario. To generate these Submissions, a matching Transmission Profile must exist.
Note: When evaluating cross reportable registrations, only shared products or product substances are considered as eligible products.
3. Investigational to Investigational (Cross Agency)
For clinical trial Cases, after generating a Submission for the Study Registration (general reporting), Vault determines whether any company Case Products, or their Substances, are being investigated or used as comparators in another Study with an Investigational registration in different jurisdictions.
If so, Vault generates Submissions for the additional Study with unique reporting destinations.
Note: When evaluating cross reportable registrations, only shared products or product substances are considered as eligible products.
When calculating reporting obligations to the PMDA on a Global Case, Vault also determines whether any Studies registered with a non-PMDA agency have Products registered with the PMDA. If so, Vault generates Submissions to the PMDA.
4. Marketing to Marketing (Cross Agency)
For postmarket Cases, after generating a Submission for a company Case Product, Vault determines whether the Substance (active ingredient) is shared by any Company Products with a Marketing Registration in different jurisdictions.
If so, Vault generates Submissions for the other company Product with unique reporting destinations.
Note: When evaluating cross reportable registrations, only shared Products or Product Substances are considered as eligible products.
5. Marketing to Investigational (Cross Agency)
For postmarket Cases, after generating a Submission for a company Case Product, Vault determines whether that Product, or its Substance (active ingredient), is being investigated or used as comparators in another Study with Investigational registrations in different jurisdictions.
If so, Vault generates Submissions for the Study with unique reporting destinations.
Note: When evaluating cross reportable registrations, only shared Products or Product Substances are considered as eligible products.
Registration Categories: Investigational vs. Marketing
A registration can be assigned the Investigational or Marketing category. You can select the Registration Type on a Study Registration or a Product Registration.
On a Registration Type of Controlled Vocabulary, the Registration Category field identifies whether a registration category is Investigational or Marketing.
The following table outlines the Vault-managed Registration Types and their Registration Category:
| Registration Type | API Name | Registration Category |
|---|---|---|
| Marketing Authorisation Application (MAA) | maa__v |
Marketing |
| Clinical Trial Application (CTA) | cta__v |
Investigational |
| New Drug Application (NDA) | nda__v |
Marketing |
| Abbreviated New Drug Application (ANDA) | anda__v |
Marketing |
| Biologic License Application (BLA) | bla__v |
Marketing |
| Investigation New Drug Application | ind__v |
Investigational |
| Premarket Approval Application (PMA) | pma__v |
Marketing |
| Premarket Notification 510k (510k) | 510k__v |
Marketing |
| Humanitarian Device Exemption (HDE) | hde__v |
Marketing |
If a Registration Type is not specified, Vault uses the following logic to classify the Registration Category for Study Cases:
- Investigational: When the Case has a Study Type of Clinical Trial or blank.
- Marketing: When the Case has a Study Type of any other value, such as Individual Patient Use or Other Study.
Note: Study Products with a Study Product Role of Investigational or Active Comparator are considered to be investigational medicinal products.
Cross Reporting Evaluation of Expectedness Rule Parameter
The Cross Reporting without Datasheet Expectedness Substitution ICSR setting determines how the Safety Reporting Rule Engine evaluates the Expected rule parameter for cross reporting scenarios where additional products are found through a Substance lookup.
Note: To use this feature, your Admin must enable Cross Reporting without Expectedness Substitution.
If this setting is enabled, the Rule Engine calculates expectedness in the same way as for general reporting (that is, without substitution based on cross reportable Datasheets). See the Reporting Rule Parameter Reference for more information on how general reporting calculates expectedness.
If this setting is not enabled, the Rule Engine uses the following logic to calculate expectedness:
- Determines if there are other Company Products with the same Substance that have an associated marketing Product Registration.
- If so, Vault evaluates expectedness using the local and core Datasheets for that Company Product.
- If there are no Product Datasheets, Vault uses the value from the Expected field on the relevant Case Assessment.
- Determines if there are clinical trial Studies where company Case Products or their Substances have an associated investigational Study Registration.
- If so, Vault evaluates expectedness using the Study Product and Study core Datasheets, if available, otherwise Vault uses the core Datasheet of the substituted Product.
- If there are no Study Product, Study, or Product core Datasheets, Vault uses the value from the Expected field on the relevant Case Assessment.
Clinical Trial Study Case Expectedness Evaluations
Vault offers several configurable options to evaluate expectedness for clinical trial study Cases. These options apply to both general and cross reporting.
Cross Reporting Substance Matching
Vault supports the following methods of Substance matching when determining the cross reporting obligations for an Agency based on Case Product Substances:
| Matching Method | Description |
|---|---|
| Exact Matching | This method is the most restrictive. The cross-reportable Product must include the exact same Substance list as the Case Product. |
| Subset Matching |
The cross-reportable Product must include all of the Substances within the Case Product. The cross-reportable Product may include additional Substances in this scenario. Subset matching includes Exact matching scenarios. |
| Partial Matching |
This method is the least restrictive. The cross-reportable Product must include at least one of the same Substances as the Case Product. Partial matching includes both Subset and Exact matching scenarios. |
Your Business Admin can set the Substance matching method for an Agency using the Cross Reporting Substance Lookup Method field.
The following sections describe each of the available Substance matching methods.
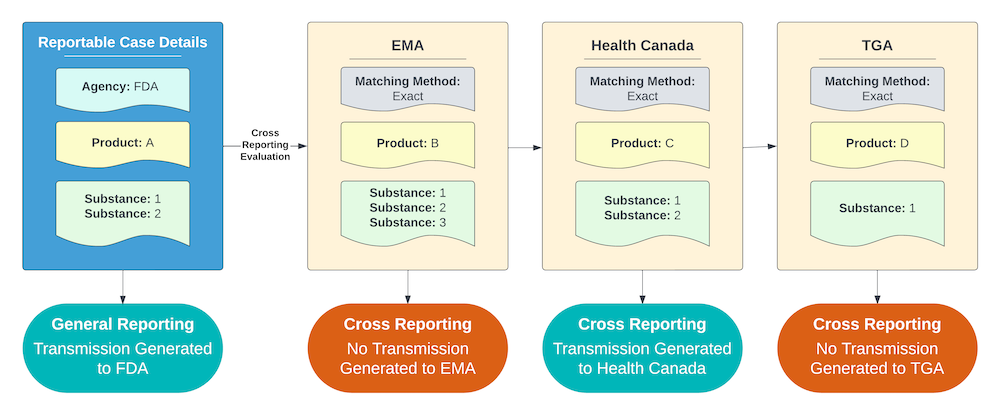
Exact Matching Method (Most Restrictive)
For an Agency using the Exact Matching method, Vault reports to the Agency if the cross-reportable Product contains the exact same Substance list as the Case Product.
The example shown below includes the following criteria:
- All of the Agencies with potentially cross-reportable Products are using the Exact Matching method.
- Product C contains the exact same Substance list as Product A, but Products B and D do not.
As a result, Vault sends a report to the Agency that Product C is registered with for any Cases containing Product A. Reports are not sent to Agencies with only Products B and D registered.
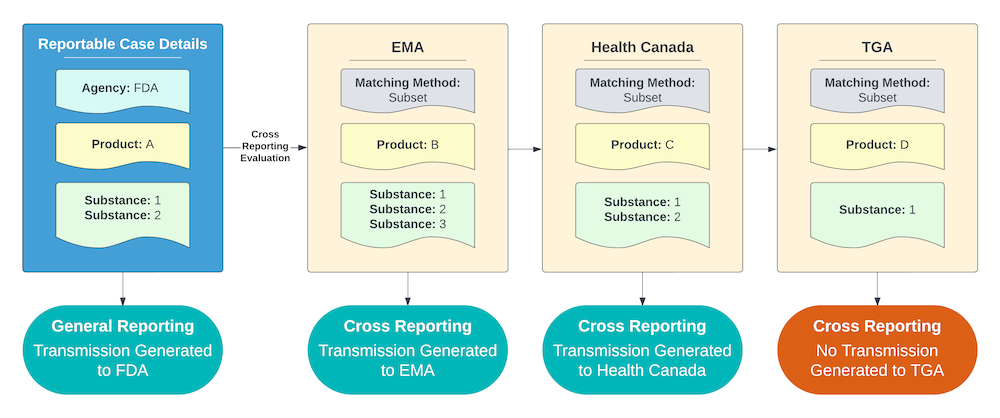
Subset Matching Method
For an Agency using the Subset Matching method, Vault reports to the Agency if the cross-reportable Product contains all of the same Substances as the Case Product.
The example shown below includes the following criteria:
- All of the Agencies with potentially cross-reportable Products are using the Subset Matching method.
- Products B and C contain all the Substances of Product A, but Product D does not.
As a result, Vault sends a report to the Agencies that Product B and C are registered with for any Cases containing Product A.
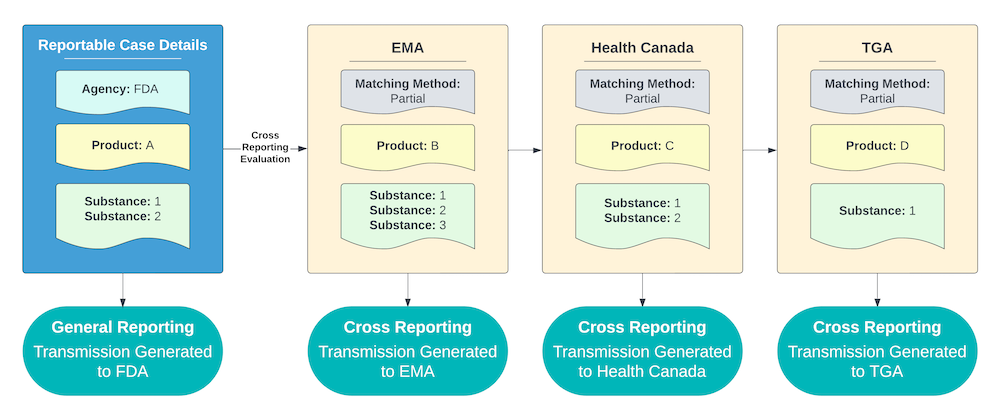
Partial Matching Method (Least Restrictive)
For an Agency using the Partial Matching method, Vault reports to the Agency if the cross-reportable Product contains at least one of the same Substances as the Case Product.
The example shown below includes the following criteria:
- All of the Agencies with potentially cross-reportable Products are using the Partial Matching method.
- Products B, C, and D each contain at least one of the Substances of Product A.
As a result, Vault sends a report to the Agencies that Product B, C, and D are registered with for any Cases containing Product A.