When you cannot submit an individual case safety report using an AS2 gateway, you can record and track manual Submissions and Distributions.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
Manual Transmissions
If you cannot submit an ICSR electronically, for example if you must submit an FDA 3500A report for a study, you can coordinate the submission manually outside of Safety.
Vault includes a Manual Transmission workflow to track past submissions and status of ongoing submissions. This page describes the default Manual Transmission workflow. However, your organization may have a custom configuration.
Note: Other than Origin and Destination, Vault Safety does automatically populate fields from the Transmission Profile on manually created Submissions or Distributions. You must populate the remaining fields (such as Message Subject and Message Body for Email Transmissions) manually in order for the system to send the Transmission successfully.
Prerequisites
- The Transmission must be created, either as a Submission or Distribution.
- To populate the sender contact details on the generated ICSR, the Sender User should have full contact and organization details on their Vault Safety account.
Manual Transmission Workflow
Note: This page describes the default Manual Transmission workflow. If your organization has a custom workflow, follow the recommendations in your business standard operating procedure.
| Stage | Lifecycle State | Description |
|---|---|---|
| Pending | Pending | Pending is the initial stage and state of the Submission record after you generate the submission document(s). The system assigns a task to review and approve the submission. |
| Pending | Ready to Submit | After the Submission record and ICSR document(s) are reviewed and approved, the Submission record enters this state to indicate that it is ready for submission. The system assigns a task to manually submit the report. |
| Accepted | Complete | After the task to manually submit the case is marked as complete, the Submission record enters the Accepted stage and the Complete state to indicate submission is done. |
Generate the ICSR Document for Submission
Generating the submission document starts the FDA Manual Submission workflow.
- Go to the Submission record.
- Check that the report type in the Transmission Document Type field is the report that you want to generate.
- To change the report type, select Edit, and then choose a different report in the Transmission Document Type field.
- Expand the All Actions menu, and then select Generate Transmission Document(s).
Result
The system generates the case report document and adds a link to the document to the File field. If you do not see the file, refresh the page.
The Submission record enters the Pending stage. The system assigns a Review Submission task to the Sender User.
Review the Submission
- Go to the Submission record.
- Review the Details section and ensure the details are correct.
- To review the submission document, in the File field, select the document link. The document opens in the Library.
- Once you have reviewed the file, return to the Submission record.
- In the Review Submission task banner, select Complete. The Review Submission window appears.
- Select Complete.
Result
The system assigns a Submit to Regulatory Authority task.
Complete the Submission
Coordinate the submission outside of Vault Safety and then mark the submission as complete to finalize the submission.
Export the Submission Document
- Go to the Submission record.
- In the File field, select the document link. The document opens in the Library.
- Expand the Document menu, and then select Source Document.
Note: The Document menu appears as  for CIOMS I and FDA 3500A reports and
for CIOMS I and FDA 3500A reports and  for E2B reports.
for E2B reports.
Result
The file starts downloading to your computer.
Mark the Submission as Complete
- Accept the Submit to Regulatory Authority task.
- Beside the Submit to Regulatory Authority task, select Complete. The Submit to Regulatory Authority window appears.
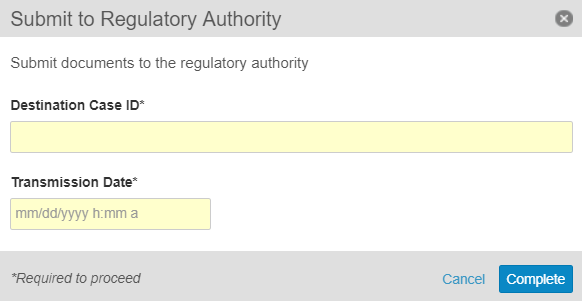
- Under Destination Case ID, enter the destination identification number for the submission.
- Under Transmission Date, enter the date and time when the report was submitted.
- Select Complete.
Result
The Submission record moves to the Completed state.