Learn how to set up PADER aggregate reports and generate PADER report tables.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
About PADER Reports
Vault Safety provides U.S. Periodic Adverse Drug Experience Report (PADER) authoring and table generation capabilities.
Note: If you are a Safety Workbench user, see Create Workbench PADER Aggregate Reports.
The following table summarizes the PADER tabulations that Vault Safety generates:
| Tabulation | Generated by Default? | Masking Support? |
|---|---|---|
| 15 Day and Non-15-Day Summary Reports | Yes | No |
| Summary of ADR from Postmarketing Sources | Yes | No |
| Interval Line Listings | Yes | No |
| Appendix: Non-Primary Suspect Product Report | Yes | No |
| Appendix: List of Death Cases | No | No |
Note: An Admin can configure custom PADER report templates for your organization.
Prerequisites
To be included in a PADER aggregate report, a Case must have a Transmission record with a Transmission Date. For more information on Case filtering, see the FDA Transmission in Reporting sections within PADER Table Generation Data Mapping.
Consider the following additional prerequisites before you generate PADER tables:
- You must be assigned permissions to view and prepare aggregate reports.
Typically, these permissions are reserved for the Safety Writer and Head of Safety roles. - Your Admin must have configured the Reporting Family with the Products and Studies to include in the report.
- Your Admin must have configured US-based Local Product Datasheets that list expected adverse events for the reporting family product.
Vault Safety uses Product Datasheets to classify adverse events as labeled or unlabeled in PADER tables. - Depending on your Admin's configuration, Case Products with the Drug Role of Drug Not Administered may be included when generating PADERs. See Enable Extend Definition of Suspect to Drug Not Administered for more information. If your Vault is not configured to include Drug Not Administered, only Case Products with the Suspect or Interacting Drug Role are included.
- To generate table data from Study-type Cases, your Admin must have configured the appropriate Study Products.
- To have the option to filter Cases by Receipt Date/New Info Date, in addition to Transmission Date, your Vault must be configured. Contact Veeva Support to enable this behavior.
- To add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report, your Admin must enable the Criteria Page for Aggregate reports.
Note: Vault Safety generates PADER tables using controlled vocabularies to determine when causality is not related. An Admin can configure these controlled vocabularies differently. Configure Controlled Vocabularies provides instructions.
Create a PADER Aggregate Report
Create a PADER Aggregate Report and specify the report settings.
Add a PADER
- In the Vault primary navigation bar, select Aggregate Reports > PADER, and then select Create. If you do not see PADER as an option, an Admin must update your Vault to Enable PADER Authoring and Table Generation.
- In the Create Aggregate Report window, under Select Aggregate Report Type, select PADER.
- Complete the fields on the Create PADER page.
- Save the page.
Result
The PADER record enters the Pending state. The system assigns a task to users in the Safety Writer role to review the report details.
PADER Fields
You can specify the following fields for a PADER Aggregate Report:
| Field | Description |
|---|---|
| Product Family (Required) | Select the Reporting Family configured for aggregate reporting.
Note: The Reporting Family object type should be Product Family.
|
| Organization | Vault populates this field with the Organization on the selected Reporting Family. |
| Data Period Start (Required) | Enter the start date for the reporting period. Vault uses the Cases within the reporting period to generate the table data. Cases are included when the date corresponding to the Filter Case By setting is within the reporting period. Cumulative reports do not consider the start date. The data period contains all Cases up to the Data Period End Date. To learn more, see How Aggregate Reports Filter by Data Period. |
| Data Period End (Required) | Enter the end date for the reporting period. To learn more, see How Aggregate Reports Filter by Data Period. |
| Filter Case By | To customize how Vault filter Cases within the specified date range, select an option:
If this field is not specified, the Case Transmission Date is used by default. |
| Include Criteria Page on Documents | Select the checkbox to add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report. When selected, the criteria page summarizes the following:
|
| States to Include (Required) |
Note: The PADER ignores most lifecycle states, with the exception of Nullified or Voided state or a lifecycle state assigned to the Deleted state type. If the Case is in one of these states and the Transmission Reason is set to Follow-Up, the Case is excluded from the PADER.
Only Cases in the Approved, Closed, Superseded, and Medical Review states are included. Note that while Superseded is not listed as an option, the Closed state includes the Superseded state. |
| Documents to Generate | You can select which documents to generate. The following options are available:
If you don't specify this field, by default Vault generates the following documents:
Depending on when your Vault was originally deployed, an Admin may need to add this field to appear on the layout. |
Generate PADER Tabulations
Review and verify the report settings. Once you have confirmed the report details are correct, use the Generate Aggregate Report Tabulations action to generate PADER report tables.
PADER Table Generation Data Mapping
Vault Safety populates aggregate report tables using Cases within the reporting period specified on the PADER, and the reporting family members configured on the associated Reporting Family.
The following sections describe how Vault Safety generates PADER tabulations:
- 15 Day and Non-15-Day Summary Reports
- Summary of ADR from Postmarketing Sources
- Interval Line Listings
- Appendix: Non-Primary Suspect Product Report
- Appendix: List of Death Cases
15 Day and Non-15-Day Summary Reports
Case-Based Report: This report prints the number of Cases that match report criteria, using the primary Case Adverse Event to categorize the Case into the appropriate column.
The 15-Day and Non-15 Day Summary Reports tables are generated for both initial reports and follow-up reports.
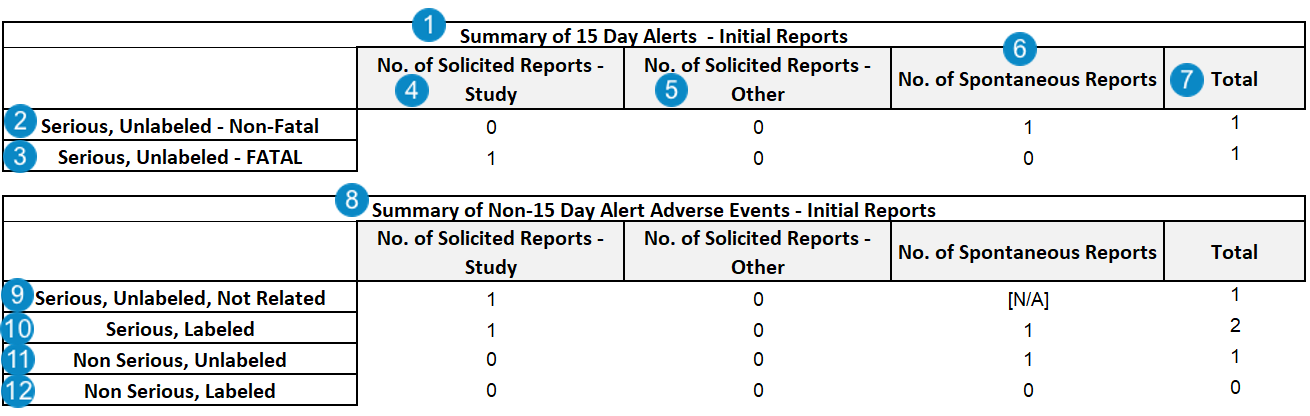
The following image shows the Summary of 15 Day and Non-15-Day Reports table. While the following image is of initial report tables, equivalent tables are generated for follow-up reports:
Table Constraints
The system filters Cases to include in the PADER 15-Day and Non-15 Day Summary Reports using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Depending on whether the PADER report is set to filter Cases by Transmission Date or Receipt Date / New Info Date, the system finds Cases submitted to the FDA within the reporting period as follows:
- Cases Filtered by Transmission Date: The system filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
- Cases Filtered by Receipt Date / New Info Date: The system filters by the New Info Date field, if available, or the Receipt Date field.
See How Aggregate Reports Filter by Data Period for more information.
Filtering Cases by Transmission Date
If filtering Cases by Transmission Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= data_period_end__v - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Filtering Cases by Receipt Date / New Info Date
If filtering Cases by Receipt Date / New Info Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is on or after the reporting period start date specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= today() - The Transmission is in any lifecycle state other than Withdrawn or Inactive, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v != (inactive_state__v OR withdrawn_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Only the latest version of the Case Transmitted to the FDA within the reporting period is listed in the report.
Note: “If the system finds a matching Transmission where the Transmission Reason field is set to “Follow-Up”, the Case is omitted if the Case is in one of the following states:
- Voided (
voided_state__v) - A Lifecycle State assigned a State Type of “Deleted”
Suspect, Interacting, or Drug Not Administered Product in Reporting Family
A Case Product must meet both of the following conditions:
- The Product field must link to a Product record added as a member of the Reporting Family
Note: For Study Products in PADER Reporting Families, you must add both the Study and Product.
case_version__vr.case_product__v.product__v IN
aggregate_report_family__vr.aggregate_report_family_join__vr.products__v - The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered.
Initial vs. Follow-Up Report
To determine whether Cases should be listed in Initial or Follow-Up Reports, the system evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial Reports: When there is at least one FDA Transmission within the reporting period with the Transmission Reason set to one of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B Code is set to
I(Initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I - Follow-Up Reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B Code is not set to
I(Initial) or1(Nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Table Mapping
Sorting: Cases are sorted in ascending order by Worldwide UID.
| Number | Name | Description | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Summary of 15 Day Alerts | The Summary of 15 Day Alerts table contains Cases with one or more FDA Transmissions within the reporting period where the Local Expedited Criteria field is set to Yes or is blank.case_version__v.transmission__v.case_expedited__v = Yes OR Blank |
|||||||||||||
| Serious, Unlabeled - Non-Fatal | The sum of all adverse events that meet the following criteria:
|
|||||||||||||
| Fatal | One (1) of the following conditions must be met to indicate a death occurred:
|
|||||||||||||
| No. of Solicited Reports - Study | Number of Cases that match one (1) of the following scenarios:
COUNT IF
|
|||||||||||||
| No. of Solicited Reports - Other | Number of Cases that match one (1) of the following scenarios:
COUNT IF
|
|||||||||||||
| No. of Spontaneous Reports | Number of adverse events with the Case > Report Type set to one (1) of the following:
case_version_v.report_type__v.controlled_vocabulary__v.e2b_code__v ≠ 2
|
|||||||||||||
| Total | The total sum of adverse events for each category. | |||||||||||||
| Summary of Non 15 Day Alerts | The Summary of Non 15 Day Alerts table contains Cases where all FDA Transmissions within the reporting period have the Local Expedited Criteria field set to No.
case_version__v.transmission__v.case_expedited__v = No |
|||||||||||||
| Serious, Unlabeled, Not Related | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
|||||||||||||
| Serious, Labeled | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
|||||||||||||
| Non Serious, Labeled | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
|||||||||||||
| Non Serious, Unlabeled | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
Summary of ADR from Postmarketing Sources
Event-Based Report: This report prints the number of Case Adverse Events that match report criteria.
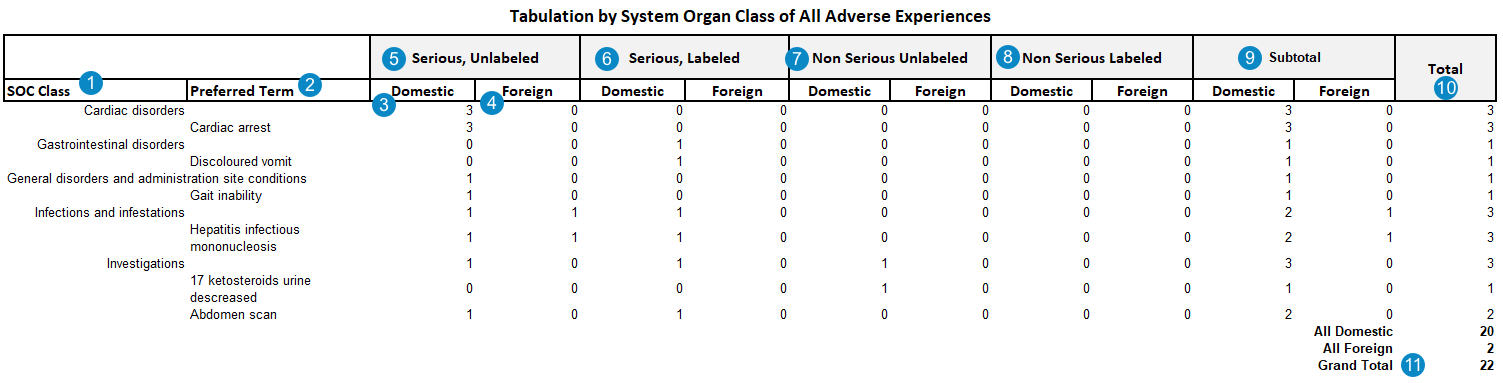
The following image shows the PADER Summary of ADR from Postmarketing Sources report:
Table Constraints
The system filters Cases to include in the PADER Summary of Adverse Drug Reactions from Postmarketing Sources using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Depending on whether the PADER report is set to filter Cases by Transmission Date or Receipt Date / New Info Date, the system finds Cases submitted to the FDA within the reporting period as follows:
- Cases Filtered by Transmission Date: The system filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
- Cases Filtered by Receipt Date / New Info Date: The system filters by the New Info Date field, if available, or the Receipt Date field.
See How Aggregate Reports Filter by Data Period for more information.
Filtering Cases by Transmission Date
If filtering Cases by Transmission Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= data_period_end__v - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Filtering Cases by Receipt Date / New Info Date
If filtering Cases by Receipt Date / New Info Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is on or after the reporting period start date specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= today() - The Transmission is in any lifecycle state other than Withdrawn or Inactive, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v != (inactive_state__v OR withdrawn_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Only the latest version of the Case Transmitted to the FDA within the reporting period is listed in the report.
Note: “If the system finds a matching Transmission where the Transmission Reason field is set to “Follow-Up”, the Case is omitted if the Case is in one of the following states:
- Voided (
voided_state__v) - A Lifecycle State assigned a State Type of “Deleted”
Suspect, Interacting, or Drug Not Administered Product in Reporting Family
A Case Product must meet both of the following conditions:
- The Product field must link to a Product record added as a member of the Reporting Family
Note: For Study Products in PADER Reporting Families, you must add both the Study and Product.
case_version__vr.case_product__v.product__v IN
aggregate_report_family__vr.aggregate_report_family_join__vr.products__v - The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered.
Note: The PADER Summary of ADR from Postmarketing Sources report only considers Case Adverse Events with Case Assessments.
Table Mapping
Sorting: Cases are sorted in ascending order, first by UID and then by Worldwide UID.
| Number | Name | Description |
|---|---|---|
| SOC Class | The MedDRA System Organ Class (SOC) for the adverse event. The sum of all adverse events under this SOC is listed under each column.
event_meddra__v.soc_term__v |
|
| Preferred Term | The MedDRA Preferred Term coded on the Case Adverse Event, grouped by the MedDRA SOC.
case_adverse_event__v.event_meddra__v.pt_term__v
Note: Contact Veeva Support to request PT Aggregation in periodic reports, which counts only unique instances of Preferred Terms (PT) in summary tabulations. Once this feature is enabled, when a Case contains multiple Case Adverse Events coded under the same MedDRA Preferred Term (PT), the report counts a single PT event instead of multiple events. |
|
| Domestic | The sum of adverse events where the Case Adverse Event > Event Country field is set to a Country with the Agency field set to FDA.
case_adverse_event__v.event_country__v.agency__v = fda__v |
|
| Foreign | The sum of adverse events where the Case Adverse Event > Event Country field is set to a Country with the Agency field not set to FDA.
case_adverse_event__v.event_country__v.agency__v ≠ fda__v |
|
| Serious, Unlabeled | Primary adverse events that meet the following criteria:
|
|
| Serious, Labeled | Primary adverse events that meet the following criteria:
|
|
| Non Serious Unlabeled | Primary adverse events that meet the following criteria:
|
|
| Non Serious Labeled | Primary adverse events that meet the following criteria:
|
|
| Subtotal | Subtotal sum of adverse events for each MedDRA SOC and PT, within domestic or foreign jurisdiction. | |
| Total | Total sum of domestic and foreign adverse events for each MedDRA SOC and PT. | |
| Grand Total | Total sum of all adverse events. |
The Cases contained in the report are listed in a separate table:
Interval Line Listings
Case-Based Report: This report prints the number of Cases that match report criteria.
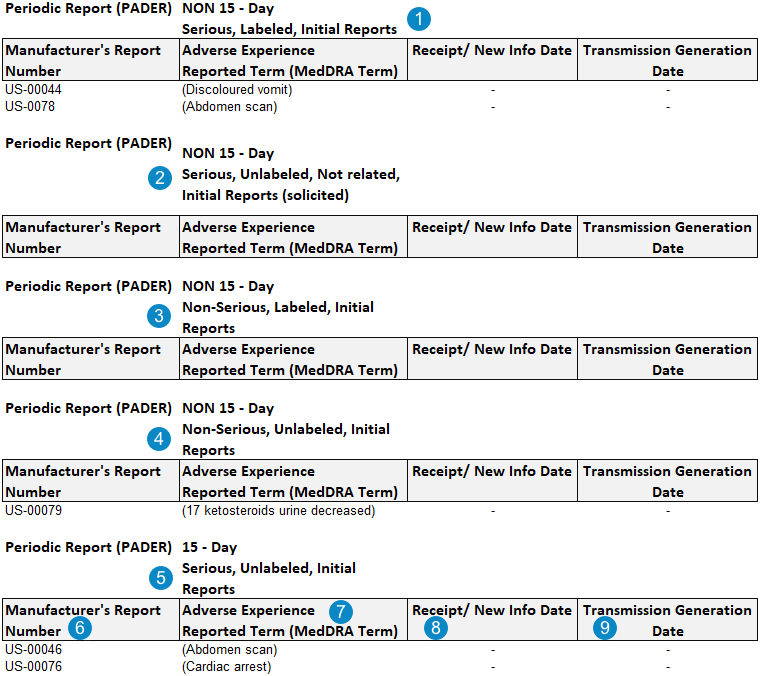
Vault Safety generates Interval Line Listings tables for both initial and follow-up reports. PADER Interval Line Listings include the following ten (10) indexes:
- Index 1: Serious, Labeled, Initial
- Index 2: Serious Unlabeled, Not Related, Initial (Solicited)
- Index 3: Non-Serious, Labeled, Initial
- Index 4: Non-Serious, Unlabeled, Initial
- Index 5: Serious, Labeled, Follow Up
- Index 6: Serious, Unlabeled, Not Related, Follow Up (Solicited)
- Index 7: Non-Serious, Labeled, Follow Up
- Index 8: Non-Serious, Unlabeled, Follow Up
- Index 9: Serious, Unlabeled, Initial (15-day)
- Index 10: Serious, Unlabeled, Follow Up (15-day)
The following image shows the PADER Interval Line Listings. While the following image is of initial report tables, equivalent tables are generated for follow-up reports:
Table Constraints
The following table describes how the system filters Cases to include in the PADER Interval Line Listings.
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Depending on whether the PADER report is set to filter Cases by Transmission Date or Receipt Date / New Info Date, the system finds Cases submitted to the FDA within the reporting period as follows:
- Cases Filtered by Transmission Date: The system filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
- Cases Filtered by Receipt Date / New Info Date: The system filters by the New Info Date field, if available, or the Receipt Date field.
See How Aggregate Reports Filter by Data Period for more information.
Filtering Cases by Transmission Date
If filtering Cases by Transmission Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= data_period_end__v - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Filtering Cases by Receipt Date / New Info Date
If filtering Cases by Receipt Date / New Info Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is on or after the reporting period start date specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= today() - The Transmission is in any lifecycle state other than Withdrawn or Inactive, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v != (inactive_state__v OR withdrawn_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Only the latest version of the Case Transmitted to the FDA within the reporting period is listed in the report.
Note: “If the system finds a matching Transmission where the Transmission Reason field is set to “Follow-Up”, the Case is omitted if the Case is in one of the following states:
- Voided (
voided_state__v) - A Lifecycle State assigned a State Type of “Deleted”
Suspect, Interacting, or Drug Not Administered Product in Reporting Family
A Case Product must meet both of the following conditions:
- The Product field must link to a Product record added as a member of the Reporting Family
Note: For Study Products in PADER Reporting Families, you must add both the Study and Product.
case_version__vr.case_product__v.product__v IN
aggregate_report_family__vr.aggregate_report_family_join__vr.products__v - The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered.
Initial vs. Follow-Up Report
To determine whether Cases should be listed in Initial or Follow-Up Reports, the system evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial Reports: When there is at least one FDA Transmission within the reporting period with the Transmission Reason set to one of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B Code is set to
I(Initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I - Follow-Up Reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B Code is not set to
I(Initial) or1(Nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: For each Case included in the report, the PADER Interval Line Listings displays all Case Adverse Events.
Table Mapping
| Number | Name | Description |
|---|---|---|
| Non 15-Day Report Serious Labeled |
|
|
| Non 15-Day Report Serious, Unlabeled, Not Related (solicited) |
|
|
| Non 15-Day Report Non Serious Labeled |
|
|
| Non 15-Day Report Non Serious Unlabeled |
|
|
| 15 Day Report Serious Unlabeled |
|
|
| Manufacturer Report Number | This value is mapped from the Case > UID field.
case_version__v.uid__v
|
|
| Adverse Experience Reported Term (MedDRA Term) | Both the reported name and the MedDRA Preferred Term for the adverse event.
(IF event_reported_english__v ≠ BLANK
|
|
| Receipt / New Info Date | If the Cases on the report are filtered by Transmission Date, the latest FDA transmission date is populated. This value is mapped from the Transmission Date field for the most recent FDA Transmission within the reporting period.
If the Cases on the report are filtered by Receipt Date / New Info Date, the New Info Date is populated. This value is mapped from the New Info Date field on the Case.
|
|
| Transmission Generation Date |
If Cases on the report are filtered by Transmission Date, this field is populated with the most recent Transmission Date for a Case Transmission that meets the following criteria:
|
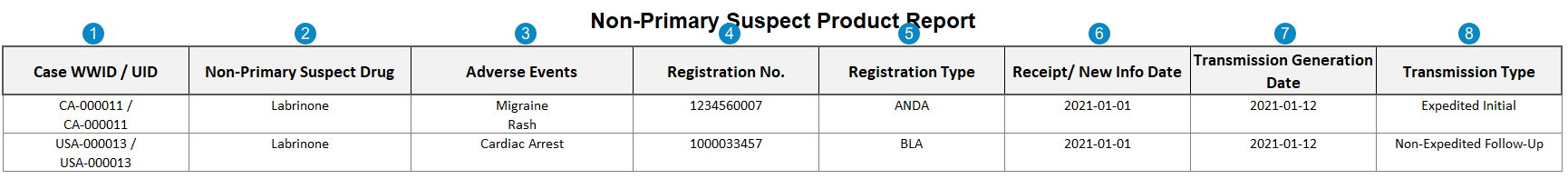
Appendix: Non-Primary Suspect Product Report
Case-Based Report: This report prints the number of Cases that match report criteria.
The Non-Primary Suspect Drug Report is a summary listing of the adverse events in which the drug or biological product was listed as one (1) of the suspect products, but the report was filed to another NDA, ANDA, or BLA held by the applicant.
Note: As of 21R1, the Non-Primary Suspect Product Report is generated for PADER reports by default. For Vaults originally deployed before 21R1, an Admin must upload the Non-Primary Suspect Product Report template, otherwise the system cannot generate this report.
Table Constraints
The system filters Cases to include in the PADER Non-Primary Suspect Product Report using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Depending on whether the PADER report is set to filter Cases by Transmission Date or Receipt Date / New Info Date, the system finds Cases submitted to the FDA within the reporting period as follows:
- Cases Filtered by Transmission Date: The system filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
- Cases Filtered by Receipt Date / New Info Date: The system filters by the New Info Date field, if available, or the Receipt Date field.
See How Aggregate Reports Filter by Data Period for more information.
Filtering Cases by Transmission Date
If filtering Cases by Transmission Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= data_period_end__v - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Filtering Cases by Receipt Date / New Info Date
If filtering Cases by Receipt Date / New Info Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is on or after the reporting period start date specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= today() - The Transmission is in any lifecycle state other than Withdrawn or Inactive, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v != (inactive_state__v OR withdrawn_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Only the latest version of the Case Transmitted to the FDA within the reporting period is listed in the report.
Note: “If the system finds a matching Transmission where the Transmission Reason field is set to “Follow-Up”, the Case is omitted if the Case is in one of the following states:
- Voided (
voided_state__v) - A Lifecycle State assigned a State Type of “Deleted”
Non-Primary Case Product in Reporting Family
The Case must contain a Case Product that meets the following conditions:
- The Product field must link to a Product record added as a member of the Reporting Family
Note: For Study Products in PADER Reporting Families, you must add both the Study and Product.
- The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4 - The Case Product must not be designated as the primary product (Primary is No and Rank does not equal 1)
case_version__vr.case_product__v.product__v
where primary__v ≠ True (Rank ≠1)
AND drug_role__v = Suspect OR Interacting OR Drug Not Administered
IN aggregate_report_family__vr.aggregate_report_family_join__vr.products__v
Table Mapping
Sorting: Cases are sorted in ascending order, first by UID and then by Worldwide UID.
| Number | Name | Description |
|---|---|---|
| Case WWID / UID | Values are mapped from the following fields:
|
|
| Non-Primary Suspect Product | The name of each non-primary suspect or interacting Case Product.
case_version__v.case_product__v.product_name__v |
|
| Adverse Events | The MedDRA PT of each Case Adverse Event, sorted by primary first.
case_adverse_event__v.event_meddra__v.pt_term__v |
|
| Registration Number | The value from the Registration Number field on the Product Registration associated with the Case Product.
case_product__v.product_registration__v.registration_number__v |
|
| Registration Type | The value from the Registration Type field on the Product Registration associated with the Case Product.
case_product__v.product_registration__v.registration_type__v |
|
| Receipt / New Info Date |
The New Info Date is populated. This value is mapped from the New Info Date field on the Case.
If Cases on the report are filtered by Receipt Date / New Info Date, this field is populated with the New Info Date for a Case with a Transmission record that meets the following criteria:
|
|
| Transmission Generation Date | If Cases on the report are filtered by Transmission Date, this field is populated with the most recent Transmission Date for a Case Transmission that meets the following criteria:
|
|
| Transmission Type | The type is determined using the Transmission Reason and Local Expedited Criteria fields on Transmissions associated with the Case.
|
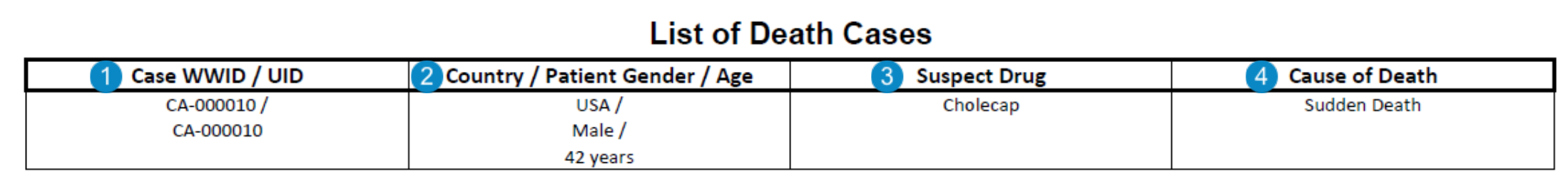
Appendix: List of Death Cases
Case-Based Report: This report prints the number of Cases that match report criteria.
The List of Death Cases for PADER lists all cases that were transmitted to the FDA during the reporting period as a 15 or non-15 day report, where the patient has died.
Note: For Vaults originally deployed before 21R1, an Admin must upload the List of Death Cases report template before you can generate this report.
Table Constraints
The system filters Cases to include in the PADER List of Death Cases using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Depending on whether the PADER report is set to filter Cases by Transmission Date or Receipt Date / New Info Date, the system finds Cases submitted to the FDA within the reporting period as follows:
- Cases Filtered by Transmission Date: The system filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
- Cases Filtered by Receipt Date / New Info Date: The system filters by the New Info Date field, if available, or the Receipt Date field.
See How Aggregate Reports Filter by Data Period for more information.
Filtering Cases by Transmission Date
If filtering Cases by Transmission Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= data_period_end__v - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Filtering Cases by Receipt Date / New Info Date
If filtering Cases by Receipt Date / New Info Date, Cases must have an associated Transmission that meets all of the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is on or after the reporting period start date specified on the Reporting Family.
transmission__v.transmission_date__v >= data_period_start__v
AND transmission__v.transmission_date__v <= today() - The Transmission is in any lifecycle state other than Withdrawn or Inactive, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v != (inactive_state__v OR withdrawn_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted
Only the latest version of the Case Transmitted to the FDA within the reporting period is listed in the report.
Note: “If the system finds a matching Transmission where the Transmission Reason field is set to “Follow-Up”, the Case is omitted if the Case is in one of the following states:
- Voided (
voided_state__v) - A Lifecycle State assigned a State Type of “Deleted”
Suspect, Interacting, or Drug Not Administered Product in Reporting Family
A Case Product must meet both of the following conditions:
- The Product field must link to a Product record added as a member of the Reporting Family
Note: For Study Products in PADER Reporting Families, you must add both the Study and Product.
case_version__vr.case_product__v.product__v IN
aggregate_report_family__vr.aggregate_report_family_join__vr.products__v - The Drug Role field must be set to Suspect (E2B Code=1), Interacting (E2B Code=3), or Drug Not Administered (E2B Code=4)
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: To include Case Products with the Drug Role of Drug Not Administered when generating PADERS, your Admin must have enabled Extend Definition of Suspect to Drug Not Administered.
Case Indicates a Death Occurred
One (1) of the following conditions must be met to indicate a death occurred:
- A value in the Case Date of Death field
case_version__v.dod_normalized__v ≠ Blank - Any Case Adverse Event Seriousness field contains Results in Death
case_adverse_event__v.seriousness__v = results_in_death__v - Any Case Adverse Event Outcome field contains Fatal
case_adverse_event__v.outcome__v = fatal - Any Case Adverse Event has a coded event under the MedDRA HLT of 10011907 (Death and Sudden Death)
case_adverse_event__v.event_meddra__v.meddra__v.hlt_code__v = 10011907 - The Case Autopsy field is set to Yes
case_version__v.autopsy_value__v = Yes
Table Mapping
Sorting: Cases are sorted in ascending order, first by UID and then by Worldwide UID.
| Number | Name | Description |
|---|---|---|
| Case WWID / UID | Values are mapped from the following fields:
|
|
| Country / Patient Gender / Age | Values from the following fields:
|
|
| Suspect Drug | The Case Product name, where the Drug Role is Suspect, Interacting, or Drug Not Administered.
case_version__v.case_product__v.product_name__v |
|
| Cause of Death | Both MedDRA PT and reported term entered on the Case > Cause of Death record.
IF case_cause_of_death__v.cause_of_death_meddra_pt__c ≠ Blank
|
Example PADER Generation
To review examples of PADER generation based on Case filtering, see Sample PADER Generation When Filtering by Transmission Date and Sample PADER Generation When Filtering by Receipt Date / New Info Date.