Vault supports Case translations to meet local submission requirements.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
About Localized Cases
To support translations and regional requirements for local submissions, Vault generates Localized Cases based on reporting requirements. When you create a Submission or Distribution for a Destination organization with a non-global Localization, Vault generates a Localized Case for the appropriate locale.
When generating Localized Cases, Vault also generates and links localized versions of several Case child objects. For example, if a domestic Case includes Parent Information, Vault generates a Localized Parent Information, mapping all the domestic Parent Information data.
In Vaults configured to Auto-select Inbox Item Localization by Reporter Country, for primary Reporter type Contacts, if the Reporter Country has a Localization record, Vault populates the Localization field on the Inbox Item. When you promote the Inbox Item to a Case, it is processed as a domestic Case and Vault generates a Localized Case.
Note: Contact Veeva Support to configure your Vault to consider the Report Type of the Inbox Item, along with the Reporter Country, when setting the Localization on the Inbox Item. See this note for more information.
To support scenarios where a global Case includes multiple documents and literature articles, you can quickly generate Localized Case Documents. For each reporting destination, your Admin can also configure document inclusion in E2B reports.
If your Admin has configured your Vault to isolate blinded Product information on Clinical Trial Study Cases, see Isolate Blinded Clinical Trial Information for additional considerations during Localized Case processing.
Note: When processing Cases that may be reportable to the PMDA, see Complete Intake and Process Cases for the PMDA for regionally specific features in Vault Safety.
Important Terms
- Global: Global refers to the main operating language of an international company. English is the only language considered global.
- Locale: The locale is determined by the country and language of regional agencies that require submissions. For example, Portuguese (Brazil).
- Localized Case: A Vault object used to prepare case translations for regional submissions and distributions.
Prerequisites
In Vaults originally deployed in 21R2 or earlier, your Admin must enable localized submissions and translation support.
In addition, your Admin should set up:
- The appropriate Product Registrations and Study Registrations.
- A Transmission Profile for a Destination organization with a non-global Localization.
- To automatically generate Localized Cases, Reporting Rules.
Note: Manually setting the Destination on a Transmission to an Organization with a non-global Localization also results in Vault generating a Localized Case.
Optional Configuration
Depending on your business process, your Admin may enable:
- Extend Definition of Suspect to Drug Not Administered: Includes Case Products with the Drug Role of Drug Not Administered when generating Localized Case Assessments. Otherwise, Localized Case Assessment generation considers only Case Products with the Drug Role of Suspect or Interacting.
- Localized Cases: Global Narrative Override on Follow-ups: Overrides localized narratives based on related global and domestic Case narratives.
- Destination-Specific Case Document Management Improvements: Offers reporting destination-specific attachment control when exporting E2B reports and fast generation of Localized Case Documents.
Auto-Translation Framework
In addition to Vault language support, you can configure your Vault to connect to Amazon Translate for translation of Case text fields. Depending on your business process, you can set up automatic or manual translation requests.
Note: When using the Auto-Translation Framework, Vault imports the translated text as received. Vault does not perform any quality checks on the content.
About Localized Case Generation
When you create a Transmission (Submission or Distribution), the Localization of the Destination determines whether Vault generates a Localized Case and for which locale. Your Admin configures localization requirements using the Localization field on Organization records in the Business Admin tab collection. When a Transmission specifies a Destination organization with a non-global Localization value, Vault generates a Localized Case.
Note: In the standard Vault template, the Localization of the PMDA (Japan) and MFDS (Korea) Organization records includes their respective locales.
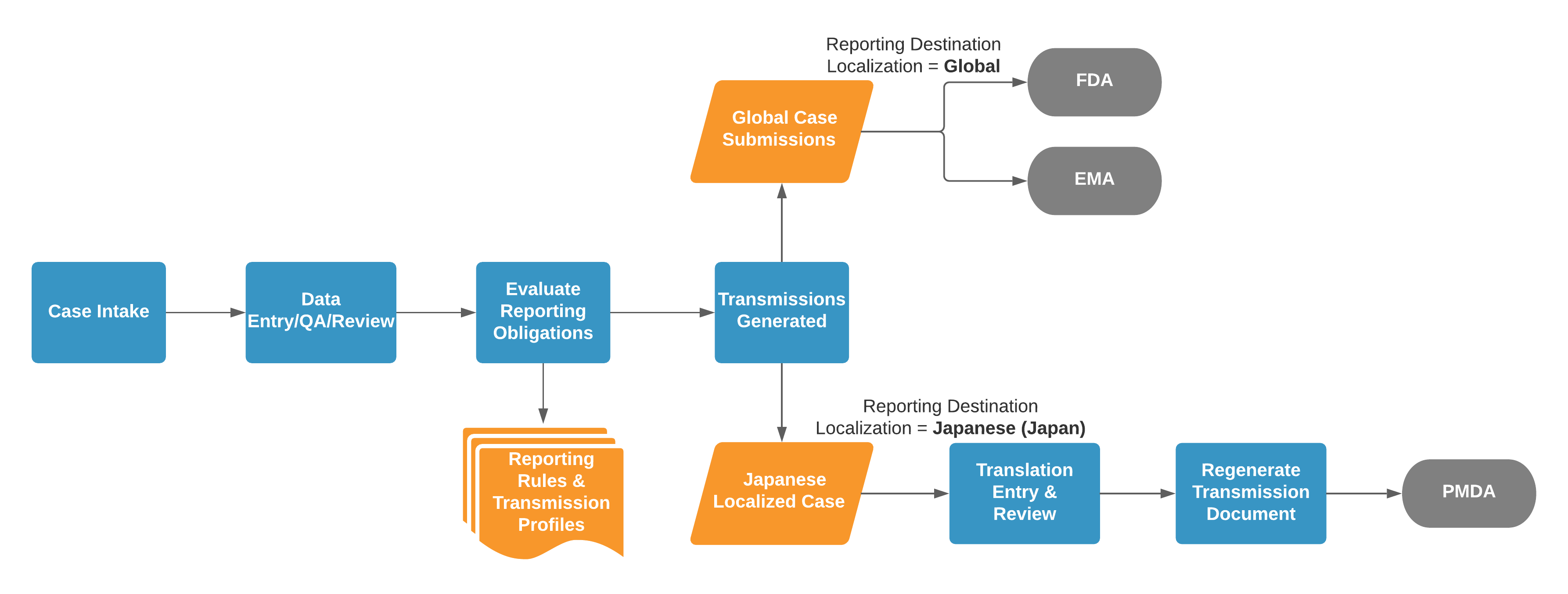
For example, consider the following process illustration where a Case matches global Transmission Profiles for the FDA and EMA, as well as a PMDA (Japan) Transmission Profile:
If two (2) Transmissions have the same target language, they both reference the same Localized Case. Vault does not generate duplicate Localized Cases with the same language.
Vault checks for localization requirements for both manually and automatically generated Transmissions. For automatic generation, the Case must trigger pre-configured reporting rules for a reporting destination.
How to Generate a Localized Case
Vault generates Localized Cases for the target languages required for submissions following Transmission generation. Generate a Transmission using one (1) of the following methods:
- Automatic: Move the Case into the Approved state or run the Evaluate Reporting Obligations action on the Case.
- Manually: Add a Submission or Distribution.
Verify the Transmission meets the following criteria:
- Destination: Specifies a Destination organization with any non-global Localization
- Transmission Document Type: Specifies a document type
- Origin and Destination: Match the Transmission Profile.
Parent Information
When a Case includes a Parent Information, Vault also generates Localized Parent Information for each Localized Case, mapping all data from and linking to the global Parent Information.
Note: Contact Veeva Support to enable deleting localized child and grandchild records when you delete global Case child records. This feature applies to domestic and foreign Localized Cases. Your Admin must then configure the Safety General Settings. As of 22R3, this enhancement requires only configuration enablement.
Evaluate Reporting Obligations for Localized Transmissions
When Localized Case processing is complete, use the Evaluate Reporting Obligation action to review reporting requirements to a Localized Case’s agency and reevaluate the due date. From the All Actions menu on a Localized Case, select Evaluate Reporting Obligations.
Vault searches for a matching reporting rule, and then regenerates and validates Submissions.
If Vault finds a matching reporting rule:
- If no Localized Cases are in progress, Vault creates a new Transmission.
- If any Localized Cases are in progress, Vault updates the Transmission field values of the Cases with the parameters of the matched reporting rule.
If Vault does not find a matching reporting rule, Vault moves all in-progress Transmissions related to the Localized Case to the Withdrawn state.
A Transmission is in progress when in any of the following states:
- Active
- Error
- Pending
- Ready for Submission
- Ready
- Validation Error
Note: If your Admin has configured your Vault to generate Localized Case Assessments, expectedness, and due dates for each Japan Case Product Registration and Case Adverse Event pair, see Complete Intake and Process Cases for the PMDA for more information.
Submit to Gateway for Localized Cases
To submit Submissions and Distributions, after processing a Localized Case, from the All Actions menu select Submit to Gateway.
Note: If Regenerate Email Transmissions When Submitting to Gateway is turned on, Vault regenerates the Transmission files before sending the Transmission.
Considerations
Be aware of the following Submit to Gateway action considerations:
- If the Validation Status of the Submission is Hard Fail, you cannot run the Submit to Gateway action. Your Admin can configure the Result Status Type to Hard Fail for certain Validation Criteria.
- In Vaults configured with Strict Transmission Version Enforcement:
- Vault does not auto-submit follow-up Submissions before the previous Submission to the same Destination (or Destination ID for the PMDA) and Transmission Profile is in the ACK Accepted or Completed state.
- If the Case has a previous version with an open Transmission to the same Destination (or Destination ID for the PMDA) and Transmission Profile, Vault asks for confirmation before sending the new Transmission.
Translating Case Data
Note: To ensure data is finalized before translation, we recommend translating Localized Cases after the initial Case moves to the Approved state.
Vault maps fields and child records from global Cases to Localized Cases as a starting point for conducting translations. On Localized Cases you can translate text fields and narratives and enter region-specific information. Changes made to Localized Cases do not impact global Cases or narratives.
Vault translates non-text fields such as units of measurement, picklists, and Controlled Vocabularies for standard supported languages. Vault does not translate fields with data that is transmitted through code in E2B, including MedDRA.
Narrative Translation
Vault generates narrative documents for the translation of Localized Cases, which includes the English narrative that exists for the related Case when you generate the Localized Case. You can review these details and access the narrative document in the Narrative section. You can override the narrative content on follow-up Localized Cases with the narrative content in the global or domestic Case. For more information about how to override localized narratives, see Generate a Case Narrative.
Supported Languages and Locales
Vault supports a number of languages by default. See About Supported Languages for the list of standard supported languages. Locale is determined using a combination of language and country. English is the only language considered global. All other languages generate Localized Case records.
Vault supports generating Localized Cases for many standard languages. If you require additional languages for translations, your administrator can update your Vault configuration to support additional locales.
Send Translation Requests Through the Auto-Translation Framework
If your organization has set up the Auto-Translation Framework, you can send Localized Case text fields to Amazon Translate for translation.
Note: Narratives and documents are not currently supported through the Auto-Translation Framework.
Your Admin can configure the Auto-Translation Framework for:
- Automated Translation Requests: Vault sends translation requests upon Localized Case generation.
- Manual Translation Requests: You send translation requests after Localized Case generation. From the All Actions menu, select Translate Localized Case.
For both automated and manual translation requests, after Vault sends the translation request, the Localized Case moves to the Translation Requested state.
Vault automatically applies generated and returned third-party translations to Localized Cases. Vault does not verify translation quality, and imports text as received. At this point, the Localized Case moves to the Translation Verification state.
Note: You can also set up the Auto-Translation Framework for intake translation using a non-AWS translation service for custom integrations. Contact Veeva Support for more information.
Considerations for Translation Requests
Be aware of the following translation request considerations:
- Localized Cases in the Translation Requested state are editable. If text fields are edited in this state, the updated values are overridden when translations are returned to Vault.
- If the length of translated text exceeds the character limit of any text field, the text is truncated to that field’s maximum length. Vault displays a notification message indicating which fields have been truncated.
- The translation must be returned within 60 minutes or the task expires. Vault displays a notification message for failed translation requests.
Translation Notifications
Depending on your Admin’s configuration, Vault may send translation notification messages in the following scenarios:
- When a translation error occurs, Vault notifies the user who sent the Localized Case for translation and moves the Localized Case to the Translation Error state. A translation error can occur if the translation is not returned to Vault within 60 minutes or if the translation service goes offline during the translation.
- When a translation is complete and pending verification, Vault notifies the user who sent the Localized Case for translation.
Translation Workflow
Refer to your organization’s standard operating procedures for details on your organization’s translation workflow.
Generate Localized Case Transmission Documents
After entering translations on a Localized Case, you can generate localized Transmissions. Navigate to the global Transmission and then from the All Actions menu, select Generate Transmission Document(s).
For more information, see Manually Generate a Submittable Report.
Manage Localized Follow-Up Cases
When you create a follow-up Case for a Localization with Local in the Localization Type field, Vault generates a domestic follow-up Localized Case. Vault copies all of the related localized field data and Localized Case records, including foreign Localized Case records, from the previous version to the domestic follow-up Localized Case. If you create the follow-up Case from an Inbox Item, Vault merges new data from the Inbox Item into the domestic follow-up Localized Case. For more information, see Default Merge Behavior.
Because Vault automatically generates follow-up Localized Cases when global follow-up Cases are created, Vault does not allow edits to follow-up Localized Cases until the associated global follow-up Case has a Transmission record. This prevents starting translations before all the information from the global Case has been entered.
Foreign Localized Case Syncing
When a global follow-up Case is automatically or manually transmitted, Vault performs a one-time data sync from the global Case to the Localized Case, as follows:
- New and edited records from the global follow-up Case are copied to the Localized Case.
- Deleted records and fields on the global follow-up Case are deleted from the follow-up Localized Case.
- Populated text fields on the Localized Case are copied to the follow-up Localized Case. If the Localized Case contains blank text fields, these fields are copied from the global follow-up Case to the follow-up Localized Case.
Note: If a follow-up Localized Case exists and has a Transmission record, Vault does not sync the data.
Localized Cases reportable to China follow separate syncing behavior.
Text Field Syncing
To enable text field syncing in your Vault, contact Veeva Support. When enabled, Vault copies text fields using one (1) of the following options:
- For populated text fields on a Localized Case, Vault copies those values to the follow-up Localized Case. For blank text fields on a Localized Case, Vault copies those values from the global follow-up Case to the follow-up Localized Case.
- For all text fields, Vault copies the values from the global follow-up Case to the follow-up Localized Case.
Regardless of your Admin’s configuration for copying text fields, Vault considers the Localization Scope for the Localization associated with the Localized Case. For example, if the Localization for Japanese (Japan) has Company Comments as its Localization Scope, Vault syncs only Company Comments field text. If the Localization Scope is blank, Vault syncs all text fields.
Localized Parent Information Updates
If a follow-up Case includes Parent Information, Vault generates or updates the corresponding Localized Parent Information and version. Vault copies all Localized Parent Information from the previous version of the record, if one exists, and all new information from the promoted Inbox Item.
Data Syncing for Localized Cases for China
When processing Localized Cases that are reportable to China, Vault maps field data from global Cases to foreign and domestic Localized Cases upon creation. Subsequently, Vault syncs global Case field data to Localized Case fields in the following instances:
- During foreign Localized Case syncing
- Following ad hoc changes to a domestic Localized Case
- Following merge to an in-flight or creation of a follow-up Case from the Inbox Item to Case Compare page on a domestic Localized Case
For the following fields, Vault syncs data from the global Case to the Localized Case only when the Localized Case field is blank or the coded Case Product has changed: