Learn how to set up PMDA Post-Market aggregate reports and generate PMDA Post-Market report tables.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
About PMDA Post-Market Reports
Vault Safety provides Pharmaceuticals and Medical Devices Agency (PMDA) Post-Market report authoring and table generation capabilities. The Vault Safety PMDA Post-Market report adheres to the PMDA Guide to Electronic Reporting of Adverse Drug Reactions and Infectious Diseases. In addition, for Japan Post-Market Serious (J-PSR) reports, Vault tracks reporting interval dates and combines data from new and previous reporting intervals to cover up to several years of information.
The following table summarizes the PMDA Post-Market tabulations that Vault Safety generates:
| Tabulation | Generated by Default? | Masking Support? |
|---|---|---|
| NUPR Line Listing Form 7-2 | Yes | No |
| J-PSR Cumulative Tabulation of Adverse Events Form 3 | No | No |
| J-PSR Line Listing of Adverse Events Form 4 | No | No |
Note: Your Admin can configure custom PMDA Post-Market report templates for your organization.
Prerequisites
Consider the following prerequisites before you generate PMDA Post-Market report tables:
- You must be assigned permissions to view and prepare aggregate reports. Typically, these permissions are reserved for the Aggregate Report Writer, Safety Writer, and Head of Safety roles.
- Your Admin must have enabled the Japan Periodic Reporting - Post-Market Non-Serious (NUPR) and Japan Periodic Reporting: Post-Market Serious (J-PSR) features.
- Your Admin must have configured the Reporting Family with the Products, Studies, Product Registrations, and Substances to include in the report.
- To add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report, your Admin must have enabled the Criteria Page for aggregate reports.
Create a PMDA Post-Market Aggregate Report
Create a PMDA Post-Market aggregate report and specify the report settings.
Add a PMDA Post-Market Report
Follow these steps to create a PMDA Post-Market report.
- In the Vault primary navigation bar, select Aggregate Reports > PMDA Post-Market, and then select Create.
- Complete the fields on the Create PMDA Post-Market page.
- Save the page.
Result
The PMDA Post-Market record enters the Pending state. Vault assigns a task to applicable users to review the report details and generates an Interval Dates section.
PMDA Post-Market Fields
You can specify the following fields for a PMDA Post-Market aggregate report:
| Field | Description |
|---|---|
| Product Family (Required) | Select the Reporting Family configured for aggregate reporting.
Note: The Reporting Family object type should be Product Family.
|
| Organization | Vault populates this field with the Organization on the selected Reporting Family. |
| Data Period Start (Required) | Enter the start date for the reporting period. Vault uses the Cases within the reporting period to generate the table data. Cases are included when the date corresponding to the Filter Case By setting is within the reporting period. Cumulative reports do not consider the start date. The data period contains all Cases up to the Data Period End Date. Vault adds this date to the Interval Start Date field of the related or current Interval Dates record. To learn more, see How Aggregate Reports Filter by Data Period. |
| Data Period End (Required) | Enter the end date for the reporting period. Vault adds this date to the Interval End Date field of the related or current Interval Dates record. To learn more, see How Aggregate Reports Filter by Data Period. |
| Previous J-PSR Report | When generating J-PSR tabulations as part of the PMDA Post-Market report, you can select a previously generated J-PSR report for that Product Family if a prior reporting interval exists. Vault copies Interval Date records from the selected report into the current report. You can view the selected report through the link in the Previous Aggregate Report field of the related Interval Dates record. If you change the Previous J-PSR Report value, Vault updates the Interval Date records based on the new selection. To learn more, see How Aggregate Reports Filter by Data Period. |
| Filter Case By | To customize how Vault filters Cases within the specified date range, select an option:
If this field is blank, Vault uses the Case Receipt Date/New Info Date. |
| Include Criteria Page on Documents | Select the checkbox to add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report. When selected, the criteria page summarizes the following:
The criteria page is in English on Japanese reports. |
| States to Include (Required) |
Select the states that Cases must be in to be included in the report. By default, only Cases in the Approved, Closed, Superseded, and Medical Review states are included. Although Superseded is not listed as an option, it is included within the Closed state. Only system-provided states in the Case Processing lifecycle are supported.
Note: If the latest Case version within the aggregate reporting period is in the Nullified or Voided state or in a lifecycle state assigned to the Deleted state type, the Case is excluded from the aggregate report.
|
| Documents to Generate | You can select which documents to generate. The following options are available:
|
Interval Dates
When you create a PMDA Post-Market report, Vault generates an Interval Dates section and populates an Interval Date record for the current report, showing the selected start and end dates for the reporting interval. If you populate the Previous J-PSR Report field when creating the report, Vault also copies all of the Interval Date records from the selected report into the current report. This supports tracking reporting cycles, which Vault uses to generate reports that combine new and previous reporting intervals to cover several years of data, per PMDA regulations.
Interval Date Fields
| Field | Description |
|---|---|
| Name | Vault generates a name for the Interval Date record. |
| Interval Start Date | Vault maps the value entered in the Date Period Start field when creating the report. If the Date Period Start value is updated, Vault updates this field with the new value. |
| Interval End Date | Vault maps the value entered in the Date Period End field when creating the report. If the Date Period End value is updated, Vault updates this field with the new value. |
| Current Interval | Vault populates "Yes" for the latest reporting interval and "No" for previous reporting intervals. When a new PMDA Post-Market report is created for a Product Family, Vault updates this value to "No" on the previous reporting interval for the Product Family. |
| Current Aggregate Report | For each Interval Date record, Vault populates a link to the current aggregate report for that Product Family's reporting cycle. Vault updates this value on all related records when a new PMDA Post-Market report is created for the Product Family. |
| Previous Aggregate Report | For each Interval Date record, Vault populates a link to the aggregate report used to define the date intervals. This field is blank for the current interval. |
Generate PMDA Post-Market Tabulations
Review and verify the report settings. Once you have confirmed the report details are correct, use the Generate Aggregate Report Tabulations action to generate PMDA Post-Market report tables.
PMDA Post-Market Table Generation Data Mapping
Vault Safety populates aggregate report tables using Cases within the reporting period specified on the PMDA Post-Market report, and the reporting family members configured on the associated Reporting Family.
The following sections describe how Vault Safety generates PMDA Post-Market tabulations:
- NUPR Line Listing Form 7-2
- J-PSR Cumulative Tabulation of Adverse Events Form 3
- J-PSR Line Listing of Adverse Events Form 4
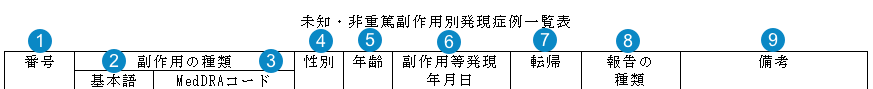
NUPR Line Listing Form 7-2
Vault generates the NUPR Line Listing Form 7-2 by default for PMDA Post-Market aggregate reports.
Table Constraints
Vault filters Cases to include in the NUPR Line Listing Form 7-2 using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
Case Product or Product Registration in Reporting Family
A Case must have a Product or Product Registration that is a member of the Reporting Family.
case_version__v.case_product__v.product__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.products__vcase_version__v.case_product_registration__v.product_registration__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.product_registrations__v
Case Data in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≤ pmda_post_market__v.data_period_end__v
where DATE depends on the option selected in the PMDA Post-Market Filter Cases By (pmda_post_market__v.filter_cases_by__v) field:
- When Approval Date:
case_version__v.approval_date__v - When Local Awareness Date:
Data Period Start Date <= localized_case_v. local_awareness_date__v <= Data Period End Date - When blank or Receipt Date / New Info Date:
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v - Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, only the most recent Case version within the reporting period is listed.
Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the PMDA Post-Market.
case_version__v.state__v CONTAINS pmda_post_market__v.states_to_include__v
Consider the following:
- Cases in the following states are always omitted and cannot be selected in the States to Include field:
- Nullified (
nullified_state__v) - Voided (
voided_state__v) - If the Case is in a Lifecycle State assigned a State Type of “Deleted”, the Case is omitted.
- When evaluating the States to Include field, Vault evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
- Nullified (
Localized Case
A Case must have a Japan localized version to be included.
case_version__v.reporter_country__v = Japan
Case Product in Drug Roles to Include
Only include Case Products in the listing where Drug Role is “Suspect” or “Interacting”.
Post-Market Cases
Only include Cases that are considered post-market.
The case_product_registration__v.registration_type_cv__v E2B code is one (1) of the following: (1, 2, 5, 6, 7)
Special Report Classification
Exclude Cases with a Special Report Classification of “Research Report” or “Safety Measure”.
case_version__v.special_report_classification__v IN (safety_measure_report__v, research_report__v)
Unexpected Adverse Events
Include only events that meet all of the following criteria:
- The event is non-serious (
localized_case_adverse_event__v.seriousness__v = Blank) - The event is Unexpected (
localized_case_assessment__v.expected__v != yes) - The event is related to the product in the report (Causality Established is set to Yes or Blank for at least one Assessment Result)
- The event is reportable (
localized_case_adverse_event__v. special_adverse_event__v != "Non-reportable Event")
Table Mapping
Sorting: Rows are in order of Event (PT) alphabetically, then by Adverse Event Onset Date (earliest first). Events with a blank Onset Date are listed last.
| Number | Name | Description |
|---|---|---|
| Case # (番号) |
The Case Numbercase_version__v.case_number__v
|
|
| Event (PT) (基本語) | The Localized MedDRA Preferred Term Event in Japanese (from the local MedDRA version) | |
| MedDRA Code (MedDRAコード) | The MedDRA Preferred Term Code | |
| Gender (性別) |
The sex on the Case If the field is blank, "不明" (Unknown in Japanese) is displayed. |
|
| Age (年齢) |
The normalized Age (in years) on the Case If age is not populated on the case, the report will display Age Group. If Age Group is not populated, it will display "不明" (Unknown in Japanese). |
|
| Onset Date (副作用等発現 年月日) |
The Onset Date of the Case Adverse Event, as entered in the Date of Onset field on the Case Adverse Event in the format "YYYY年MM月DD日" If the field is blank, "不明" (Unknown in Japanese) is displayed. |
|
| Outcome (転帰) |
The Outcome of the Case Adverse Event The value selected in the Case Adverse Event Outcome field. |
|
| Report Type (報告の 種類) |
The Case Report Type The value selected in the Case Report Type field. |
|
| Remarks (備考) |
Up to three (3) items are displayed in this column:
|
How Vault Calculates Expectedness Changes for Adverse Events on the J-NUPR-Line Listing Form 7-2
To determine if the expectedness of a Case Adverse Event has changed on the J-NUPR-Line Listing Form 7-2, Vault considers the product’s Local Datasheet for Japan. If none exists, Vault considers the product’s Core Datasheet. When no datasheets exist, Vault evaluates the Case Adverse Event as Unexpected.
For more information on expectedness evaluations, see How Vault Evaluates Expectedness.
J-PSR Cumulative Tabulation of Adverse Events Form 3
The following image map shows how Vault generates the J-PSR Cumulative Tabulation of Adverse Events Form 3 table.
Table Constraints
Vault filters Cases to include in the J-PSR Cumulative Tabulation of Adverse Events Form 3 using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
Case Product or Product Registration in Reporting Family
A Case must have a Product or Product Registration that is a member of the Reporting Family.
case_version__v.case_product__v.product__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.products__vcase_version__v.case_product_registration__v.product_registration__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.product_registrations__v
Case Data in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
Note: Unlike other aggregate reports, the PMDA Post-Market report considers the start and end dates based on the Interval Dates record instead of the Aggregate Report record.
earliest (aggregate_report_inervals__v)) ≤ DATE ≤ aggregate_report_intervals__v. latest(interval_end_date__v)
where DATE depends on the option selected in the PMDA Post-Market Filter Cases By (pmda_post_market__v.filter_cases_by__v) field:
- When Approval Date:
case_version__v.approval_date__v - When Local Awareness Date:
Data Period Start Date <= localized_case_v. local_awareness_date__v <= Data Period End Date - When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v - Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, Vault lists only the most recent Case version.
Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the PMDA Post-Market.
case_version__v.state__v CONTAINS pmda_post_market__v.states_to_include__v
Consider the following:
- Cases in the following states are always omitted and cannot be selected in the States to Include field:
- Nullified (
nullified_state__v) - Voided (
voided_state__v) - If the Case is in a Lifecycle State assigned a State Type of “Deleted”, the Case is omitted.
- When evaluating the States to Include field, Vault evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
- Nullified (
Localized Case
A Case must have a Japan localized version to be included.
case_version__v.reporter_country__v = Japan
Case Product in Drug Roles to Include
Include only Case Products in the listing where Drug Role is “Suspect” or “Interacting”.
Post-Market Cases
Include only Cases that are considered post-market and domestic.
AE > Case > Case Product Registration > Local Reporting Details > PMDA Reporting Category in:
{"AA: Post-Marketing Domestic Infection", → E2B code AA"AB: Post-Marketing Domestic AE"} → E2B code AE
Special Report Classification
Exclude Cases with a Special Report Classification of “Research Report” or “Safety Measure”.
case_version__v.special_report_classification__v IN (safety_measure_report__v, research_report__v)
Blind Setting
For Study Cases, only unblinded data is included in the report.
If your Admin has configured your Vault to isolate blinded clinical trial information, the Blinding Type on the Japan Case Product Registration must be set to Unblinded, Open, or blank.
case_product_registration__v.blinding_type__v != Blinded
Unexpected Adverse Events
All Adverse Events that are Serious and Related are included. In addition, events that meet all of the following criteria are included:
- The Localized Case Adverse Event is Serious (
localized_case_adverse_event__v.seriousness__v = !Blank) - The event is related to the Product in the report (Causality Established is set to Yes or Blank for at least one Assessment Result)
- The event is reportable (
localized_case_adverse_event__v. special_adverse_event__v != "Non-reportable Event") - The Localized Case Assessment is for a Case Product or Case Product Registration that is in the Reporting Family
case_version__v.case_product__v.product__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.products__vcase_version__v.case_product_registration__v.product_registration__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.product_registrations__v
Vault excludes Adverse Events with no Assessment or Assessment Result records.
Reporting Interval Assignments
Vault includes Cases within a reporting interval when the applicable Case date (Receipt Date / New Info Date or Approval Date) is on or between the Interval Start Date and Interval End Date on the Interval Dates record.
Cases that do not fall into any reporting intervals are mapped to the Unknown column of the report.
Table Mapping
Sorting: Rows are in order of Event (SOC) alphabetically, then Event (PT) alphabetically. Each reporting interval appears in a separate column.
| Number | Name | Description |
|---|---|---|
| Event (SOC) (器官別大分類) | The Localized MedDRA System Organ Class (SOC) affected by the Adverse Event in Japanese (from the local MedDRA version) | |
| Event (PT) (基本語) | The Localized MedDRA Preferred Term Event in Japanese (from the local MedDRA version) | |
| The Number of Adverse Events Reported (副作用・感染症の症例報告を行った症例数) | The number of reported Adverse Events for that reporting interval. A report can include mulitple reporting intervals. The reporting interval dates are included in the column headers of the report. |
|
| Unknown (不明 ) | The number of reported Cases that do not fall within the dates of any reporting interval on the report. This column appears only when the report interval dates have been set up incorrectly. |
|
| Total During Reexamination Period (再審査期間中の合計) | The total number of Cases for the reexamination period for each row on the report. | |
| Remarks (備考) | Up to two (2) items are displayed in this column:
|
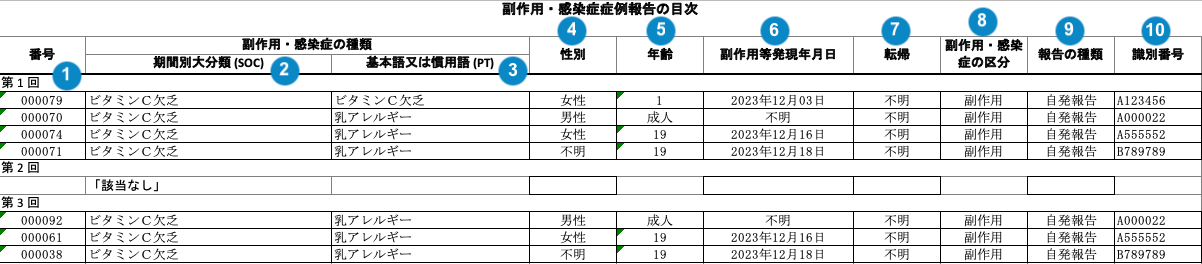
J-PSR Line Listing of Adverse Events Form 4
The following image map shows how Vault generates the J-PSR Line Listing of Adverse Events Form 4 table.
Table Constraints
Vault filters Cases to include in the J-PSR Cumulative Tabulation of Adverse Events Form 3 using the following constraints:
Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
Case Product or Product Registration in Reporting Family
A Case must have a Product or Product Registration that is a member of the Reporting Family.
case_version__v.case_product__v.product__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.products__vcase_version__v.case_product_registration__v.product_registration__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.product_registrations__v
Case Data in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
Note: Unlike other aggregate reports, the PMDA Post-Market report considers the start and end dates based on the Interval Dates record instead of the Aggregate Report record.
earliest (aggregate_report_inervals__v)) ≤ DATE ≤ aggregate_report_intervals__v. latest(interval_end_date__v)
where DATE depends on the option selected in the PMDA Post-Market Filter Cases By (pmda_post_market__v.filter_cases_by__v) field:
- When Approval Date:
case_version__v.approval_date__v - When Local Awareness Date:
Data Period Start Date <= localized_case_v. local_awareness_date__v <= Data Period End Date - When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v - Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, Vault lists only the most recent Case version.
Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the PMDA Post-Market.
case_version__v.state__v CONTAINS pmda_post_market__v.states_to_include__v
Consider the following:
- Cases in the following states are always omitted and cannot be selected in the States to Include field:
- Nullified (
nullified_state__v) - Voided (
voided_state__v) - If the Case is in a Lifecycle State assigned a State Type of “Deleted”, the Case is omitted.
- When evaluating the States to Include field, Vault evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
- Nullified (
Localized Case
A Case must have a Japan localized version to be included.
case_version__v.reporter_country__v = Japan
Case Product in Drug Roles to Include
Include only Case Products in the listing where Drug Role is “Suspect” or “Interacting”.
Post-Market Cases
Include only Cases that are considered post-market and domestic.
AE > Case > Case Product Registration > Local Reporting Details > PMDA Reporting Category in:
{"AA: Post-Marketing Domestic Infection", → E2B code AA"AB: Post-Marketing Domestic AE"} → E2B code AE
Special Report Classification
Exclude Cases with a Special Report Classification of “Research Report” or “Safety Measure”.
case_version__v.special_report_classification__v IN (safety_measure_report__v, research_report__v)
Blind Setting
For Study Cases, only unblinded data is included in the report.
If your Admin has configured your Vault to isolate blinded clinical trial information, the Blinding Type on the Japan Case Product Registration must be set to Unblinded, Open, or blank.
case_product_registration__v.blinding_type__v != Blinded
Unexpected Adverse Events
All Adverse Events that are Serious and Related are included. In addition, events that meet all of the following criteria are included:
- The Localized Case Adverse Event is Serious (
localized_case_adverse_event__v.seriousness__v = !Blank) - The event is related to the Product in the report (Causality Established is set to Yes or Blank for at least one Assessment Result)
- The event is reportable (
localized_case_adverse_event__v. special_adverse_event__v != "Non-reportable Event") - The Localized Case Assessment is for a Case Product or Case Product Registration that is in the Reporting Family
case_version__v.case_product__v.product__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.products__vcase_version__v.case_product_registration__v.product_registration__v IN aggregate_report_family__vr.aggregate_report_family_join__vr.product_registrations__v
Vault excludes Adverse Events with no Assessment or Assessment Result records.
Reporting Interval Assignments
Vault includes Cases within a reporting interval when the applicable Case date (Receipt Date / New Info Date or Approval Date) is on or between the Interval Start Date and Interval End Date on the Interval Dates record.
Cases that do not fall into any reporting intervals are mapped to the Unknown column of the report.
Table Mapping
Sorting: Rows are in order of reporting interval (earliest first), Event (SOC) alphabetically, Event (PT) alphabetically, New Info Date (earliest first), and then Case Number. If the New Info Date is blank, Vault references the Case Receipt Date (earliest first).
Each Reporting Interval heading appears on a separate line. If an interval has no Event PTs, Vault adds the following note:「該当なし」(“N/A” in Japanese).
| Number | Name | Description |
|---|---|---|
| Reporting Cycle # (番号) | The name of the reporting interval on the Interval Dates record.aggregate_intervals__vr.name__v |
|
| Event (SOC) (器官別大分類) | The Localized MedDRA System Organ Class (SOC) affected by the Adverse Event in Japanese (from the local MedDRA version). | |
| Event (PT) (基本語) | The Localized MedDRA Preferred Term Event in Japanese (from the local MedDRA version). An asterisk (*) is prepended to unexpected Adverse Events. |
|
| Gender (性別) | The sex on the Case. If the field is blank, Vault populates "不明" (Unknown in Japanese). |
|
| Age (年齢) | The normalized Age (in years) on the Case. If age is not populated on the Case, the report displays Age Group. If the field is blank, Vault populates "不明" (Unknown in Japanese). |
|
| Onset Date (副作用等発現 年月日) | The Onset Date of the Case Adverse Event, as entered in the Date of Onset field on the Case Adverse Event in the format "YYYY年MM月DD日". If the field is blank, Vault populates "不明" (Unknown in Japanese). |
|
| Outcome (転帰) | The Outcome of the Case Adverse Event. The value in the Case Adverse Event Outcome field. If the field is blank, Vault populates "不明" (Unknown in Japanese). |
|
| Infection or Adverse Event (副作用・感染症の区分) | Vault references the Localized Case Adverse Event to populate this data. If the Special Adverse Event field is set to "Infection", Vault populates 感染症 (Infection in Japanese). Otherwise, Vault populates 副作用 (Adverse Event in Japanese). |
|
| Report Type (報告の 種類) | The Case Report Type. The value in the Report Type field. |
|
| PMDA ACK # (識別番号) | The Destination ID on the Transmission. |