Vault evaluates expectedness for each Case Assessment on a Case.
About Expectedness
Upon assessment generation, Vault evaluates the expectedness of Event (LLTs) on Case Adverse Events based on the corresponding product family, product core, local, study core, and study product Datasheets. Evaluation results appear on Case Assessment Expectedness records. Vault then evaluates expectedness across all assessments to determine the expectedness for the Case.
Depending on your Admin’s configuration, Vault can also copy the justification for adverse event expectedness evaluations from Datasheets to Case Assessment Expectedness and Localized Case Assessment records. Vault also includes configurable options for evaluating expectedness on clinical trial study Cases.
Vault generates Case Assessment Expectedness records for each of the following Datasheet sources associated with the Case Product in the Case Assessment.
| Source | Expectedness Generation |
|---|---|
| Product Family Datasheet | If an assessment's Case Product inherits a Datasheet from the Product Family, Vault generates a Case Assessment Expectedness record for the Datasheet. |
| Product Core Datasheet | If an assessment's Case Product has a core Datasheet, Vault generates a Case Assessment Expectedness record for the core Datasheet. |
| Study Core Datasheet | When the assessment's Case Product is of the type Study Product, and if the Study Product Role is Investigational, Vault generates a Case Assessment Expectedness record for the Study's core Datasheet. |
| Local Datasheets | Vault generates Case Assessment Expectedness records for each Case Product Registration with a local Datasheet. Admins can configure local Datasheet to inherit listed events from the core Datasheet. |
| Study Product Datasheet | When the assessment's Case Product is of the type Study Product, Vault generates a Case Assessment Expectedness record for the study product Datasheet. Admins can configure study product Datasheets to inherit listed events from the study core Datasheet. |
| Study Product Placeholder Datasheet | When Study Product Placeholders are associated with Datasheets, Vault generates Case Assessment Expectedness records for blinded Study Products on Cases for double-blinded clinical trial Studies without Study Arms. |
Vault links all Case Assessment Expectedness records to the source Datasheet and the latest version of the referenced datasheet document in the Library.
Note:
- When assessing Combination Products, the Case expectedness is based on the drug or biologic constituent. Vault does not evaluate device constituent expectedness.
- In Vaults not configured with Datasheet Expectedness for Blinded Study Products, for blinded Cases associated with double-blinded Studies, Vault does not generate Case Assessment Expectedness records while Study Products are blinded, therefore roll-ups do not occur. In this scenario, after unblinding a Case Product, Vault generates Case Assessment Expectedness records and roll-ups occur.
About Matching Adverse Events to Datasheet Terms
To evaluate adverse event expectedness, Vault first checks whether the Event (LLT) on a Case Adverse Event matches a MedDRA Term on the Datasheets related to the Case Product or Case Product Registration. To be considered a match:
- For product core and study core Datasheets, the Event (LLT) must be listed on or fall under the hierarchy of a MedDRA Term on the Datasheet.
-
For local and study product Datasheets, Vault checks whether the Event (LLT) is listed on or falls under the hierarchy of a MedDRA Term on a Datasheet using the following priority:
- The local or study product Datasheet
- The product core or study core Datasheet
Vault also:
- Performs additional checks based on the precise expectedness and conditional expectedness settings on the Datasheet.
- Evaluates the expectedness of Event (LLTs) without specified demographics, with specified demographics, and for clinical trial study Cases.
Expectedness Evaluations Without Demographics
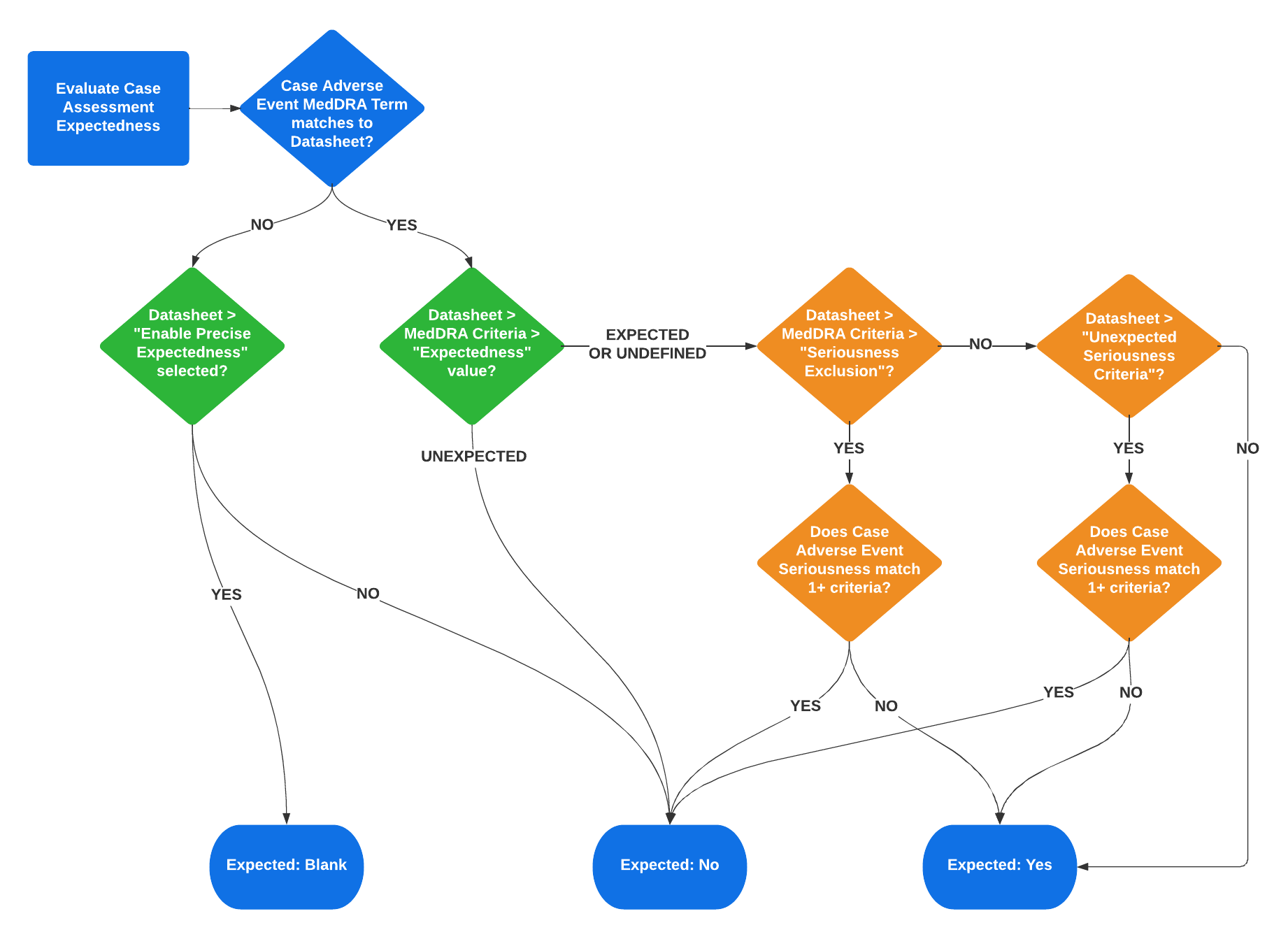
The following flowchart illustrates how Vault evaluates Case Assessment Expectedness. The green components show the precise expectedness evaluation, while the orange components show the conditional expectedness evaluation:
The following table outlines the different expectedness scenarios, based on the Datasheet settings and MedDRA Criteria settings for the event term:
| Term Matched on Datasheet? | Datasheet | MedDRA Criteria | Case Assessment Expectedness | ||
|---|---|---|---|---|---|
| Precise Expectedness Enabled? | Unexpected Seriousness Criteria | Expectedness | Seriousness Exclusion | ||
| Yes | N/A | Blank | Expected 1 | Blank | Yes |
| Yes | N/A | N/A2 | Expected | Yes: not matching 3 | Yes |
| Yes | N/A | Yes: not matching 3 | Expected | Blank | Yes |
| Yes | N/A | N/A2 | Expected | Yes: matching 3 | No |
| Yes | N/A | Yes: matching 3 | Expected | Blank | No |
| Yes | N/A | N/A | Unexpected | N/A | No |
| No | No | N/A | N/A | N/A | No |
| No | Yes | N/A | N/A | N/A | Blank |
| 1. If "Expectedness" is not defined, the value defaults to Expected. | |||||
| 2. When "Seriousness Exclusion" is defined on a MedDRA Criteria record, this setting takes precedence and the Datasheet's "Unexpected Seriousness Criteria" setting is ignored. | |||||
| 3. Matching refers to whether the Case Adverse Event has one (1) or more Seriousness values that match a Seriousness condition defined on the Datasheet or MedDRA Criteria. | |||||
Expectedness Evaluations With Demographics
Expectedness evaluations in Vault support age and sex criteria. This enables creating more precise product Datasheets for adverse events that affect specific demographics.
When matching patient details to MedDRA Criteria terms on Datasheets, Vault prioritizes the Expected/Unexpected Event Terms record with the most details. For example, if a Datasheet includes the same MedDRA Criteria term, once with sex as the only demographic detail and once with sex and age range specified, Vault matches Case Adverse Events using the second, more detailed, record.
When matching based on sex, if the Case specifies the Patient’s sex, Vault matches to MedDRA Criteria terms on the Datasheet that include that sex if such a record exists. If the MedDRA Criteria term appears on the Datasheet without a specified sex, Vault matches if all other criteria match the details on the Case.
When matching based on age, Vault matches the patient’s age to the age range of the adverse event on the Datasheet using the following priority order:
- Age at Onset calculated using the Onset date of the Case Adverse Event and the Date of Birth in the Patient section. If those values are not available, Vault maps from the manually populated Age at Onset in the Patient section.
If the Age at Onset value uses Decades as the unit, Vault considers both the lower and upper bounds of the decade when evaluating expectedness. For example, if the patient’s age is entered using2in the Number field and Decade in the Unit field, indicating they are between 10–20 years old, and the adverse event on the Datasheet is expected only for patients with an age range of 2–12 years old, Vault considers this adverse event unexpected since the upper bound of the decade (age 20) is outside the expected age range. - Age Group based on the Age Group Controlled Vocabulary type. When matching is based on Age Group, the Patient Age Group must fall entirely within the age range on the Datasheet. The following table provides examples of when Vault matches the Patient Age Group to the age range of the adverse event on the Datasheet.
| Patient Age Group (Controlled Vocabulary Age Range) |
Datasheet Age Range | Expectedness Match? |
|---|---|---|
| Child (2–11 years old) | Age Range Start: 2 years Age Range End: 20 years |
Matched |
| Adolescent (12–17 years old) | Age Range Start: 5 years Age Range End: 15 years |
Not matched |
| Adolescent (12–17 years old) | Age Range Start: 5 years Age Range End: 20 years |
Matched |
| Adult (18–64 years old) | Age Range Start: 35 years Age Range End: 55 years |
Not matched |
| Elderly (65+ years old) | Age Range Start: 60 years Age Range End: blank |
Matched |
Expectedness Evaluations for Clinical Trial Study Cases
Learn more about expectedness evaluations for clinical trial study Cases.
How-To Videos: Understanding Expectedness Evaluations
Watch our quick video tutorials on Understanding Expectedness Evaluations and Understanding Precise Expectedness Evaluations.
When Vault Evaluates Expectedness
Vault evaluates expectedness upon Case creation and aggregate report generation. Vault reevaluates expectedness when any of the following related fields are updated on a Case record:
- Case Assessment
- Case Adverse Event
- Case Product
- Case Patient Sex
- Case Patient Age (including Date of Birth, Age at Onset, or Age Group)
For clinical trial study Cases, expectedness is also reevaluated when the related Case Adverse Event Onset date is updated. If your Admin has enabled Datasheet Expectedness for Blinded Study Products, Vault also reevaluates expectedness when you modify the Blinded Name field on a Case Product.
Depending on your Admin’s configuration, Vault can always reevaluate expectedness based on follow-up information.
When reevaluating expectedness, Vault replaces the previous system-generated Case Assessment Expectedness records with new records.
Note: Case Assessment Expectedness records with an Overridden status are not deleted and do not get recreated, unless an associated Product or Adverse Event is updated.
The following table provides more information:
| Object or Field | Conditions |
|---|---|
| Case | Vault evaluates expectedness upon Case promotion. For clinical trial study Cases, Vault also reevaluates expectedness if the Adverse Event Onset Date changes. Note: To evaluate expectedness for an E2B-imported Case, products in the E2B file must be matched to a preconfigured Company Product. Expectedness is not evaluated for the Imported Case object type. |
| Case Assessment | Vault evaluates expectedness when a Case Assessment is created or updated. |
| Case Adverse Event | Vault evaluates expectedness when a Case Adverse Event related to a Case Assessment is created or either of the following fields are modified:
|
| Case Product | Vault evaluates expectedness when a Case Product with an eligible Drug Role related to a Case Assessment is created or the linked Product or Study Product is updated. If your Admin has enabled Datasheet Expectedness for Blinded Study Products, Vault also reevaluates expectedness when you modify the Blinded Name field. |
| Case Product Indication | Vault evaluates expectedness when an Indication under the Case Product related to a Case Assessment is created or the Indication (Reported) field is updated. |
| Case Patient Sex | Vault evaluates expectedness when the patient's Sex is added, changed, or deleted. |
| Case Patient Age | Vault evaluates expectedness when any of the following age-related fields on the Case are added, updated, or deleted:
|
Follow-Up Information Expectedness Reevaluation
Your Admin’s configuration of the Recalculate Post-Market Expectedness on Follow-up application setting determines when Vault reevaluates the expectedness of adverse events on postmarket Cases. This includes when Inbox Item information is merged into an in-flight Case or promoted to a follow-up Case. This setting also impacts expectedness evaluations on Cases reportable to the PMDA.
If the setting is clear, Vault copies Case Assessment Expectedness records from the previous Case version. Expectedness is not reevaluated unless the new information changes any of the following:
- Event (Coded) or Seriousness on the Case Adverse Event
- Age at Onset or Sex on the Case Patient
- Product (Coded) or Indication on the Case Product
If the setting is selected:
- For all Case Assessment Expectedness records on which the Expected (status) is Auto Calculated or blank, Vault reevaluates expectedness.
- Vault generates any new or missing Case Assessment Expectedness records for Case Products in Case Assessments.
- Vault maps global Case Assessment Expectedness updates to Localized Case Assessments as follows:
- For expectedness evaluations based on local Datasheets, when the Country on the local Datasheet matches the Country of the Localized Case, Vault maps Expected field values for which Expected (status) is Overridden, Auto Calculated, or blank.
- For expectedness evaluations based on core Datasheets, Vault maps Expected field values for which Expected (status) is Overridden, Auto Calculated, or blank. If multiple Expected values apply, Vault maps the value with the latest Last Modified Date.
- Otherwise, Vault maps the Expected value from the associated Case Assessment.