Vault uses Expectedness records to populate the Expectedness and Listedness fields on a Case.
How Vault populates these fields depends on the Datasheets available, and whether the Case is associated with a Study. The following section provides more information on the auto-calculation logic for Study Cases and non-Study Cases.
The Listedness (Status) and Expectedness (Status) fields become “Auto-Calculated” when Vault automatically populates these fields. Users can override the field values manually.
Expectedness Roll-Up Evaluation
The following illustrations show the auto-expectedness process for a Case using the Product, Study, or Study Product Datasheets:
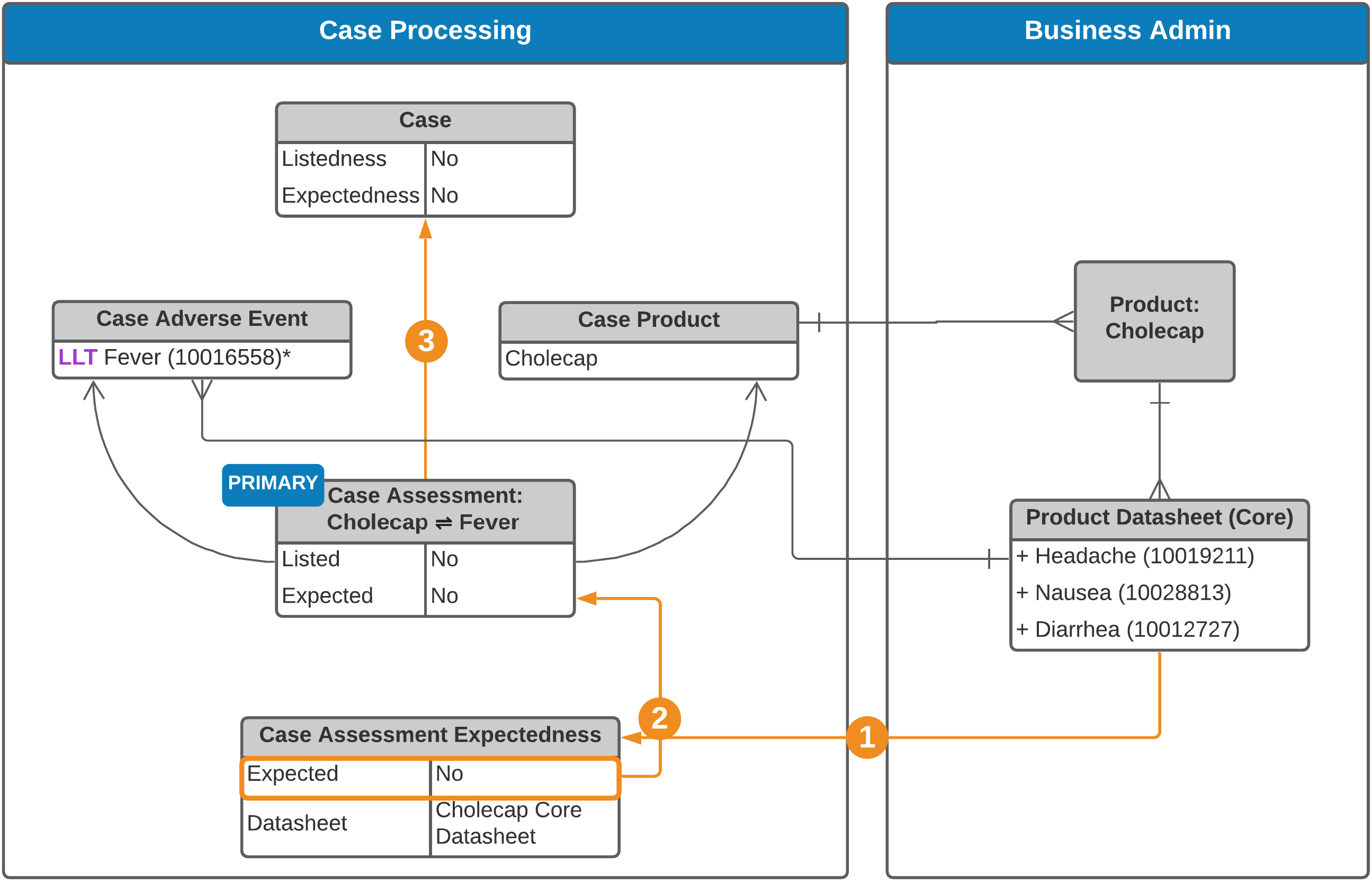
Non-Study Case
See an explanation of the steps below.
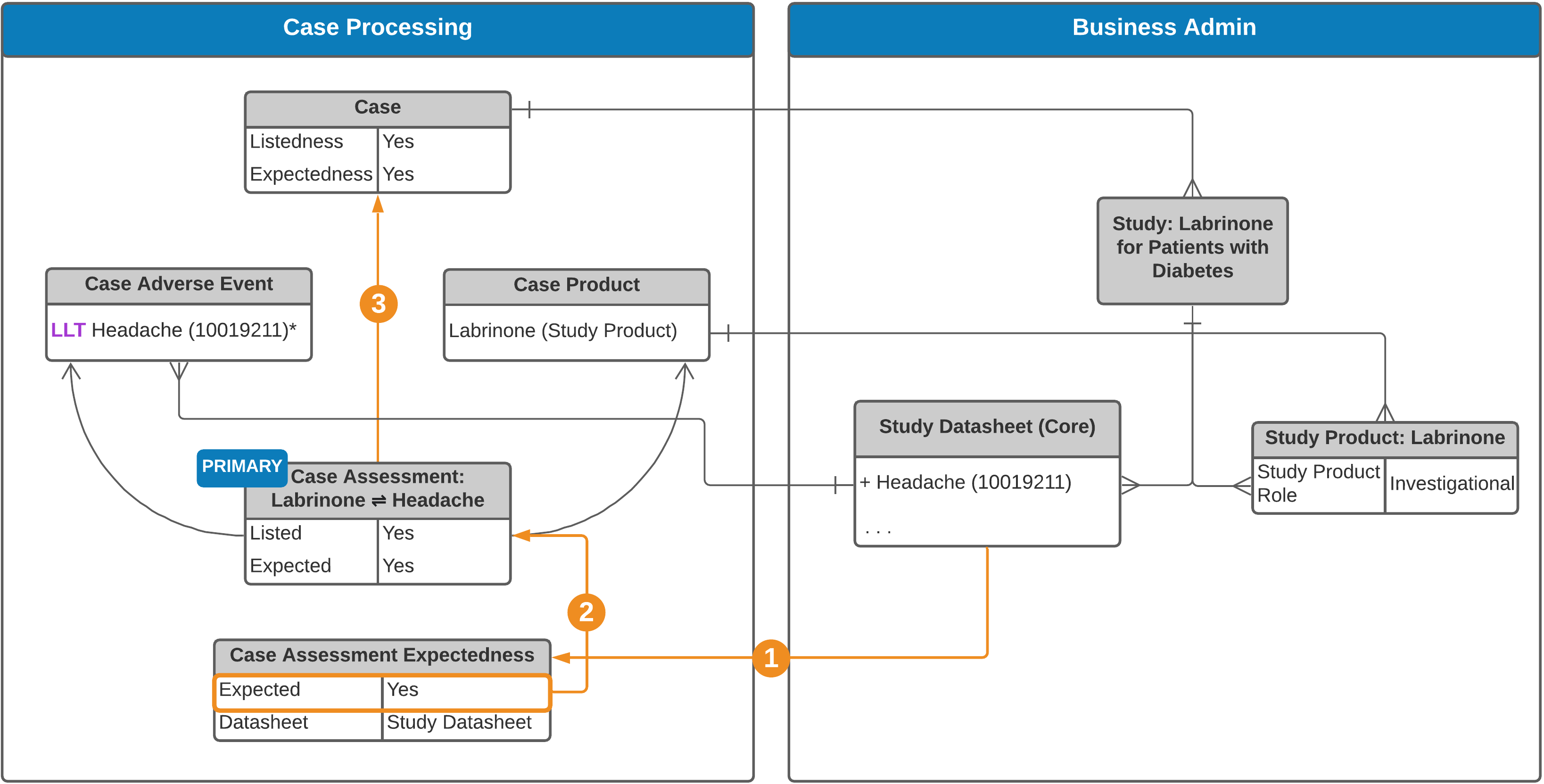
Study Case
See an explanation of the steps below.
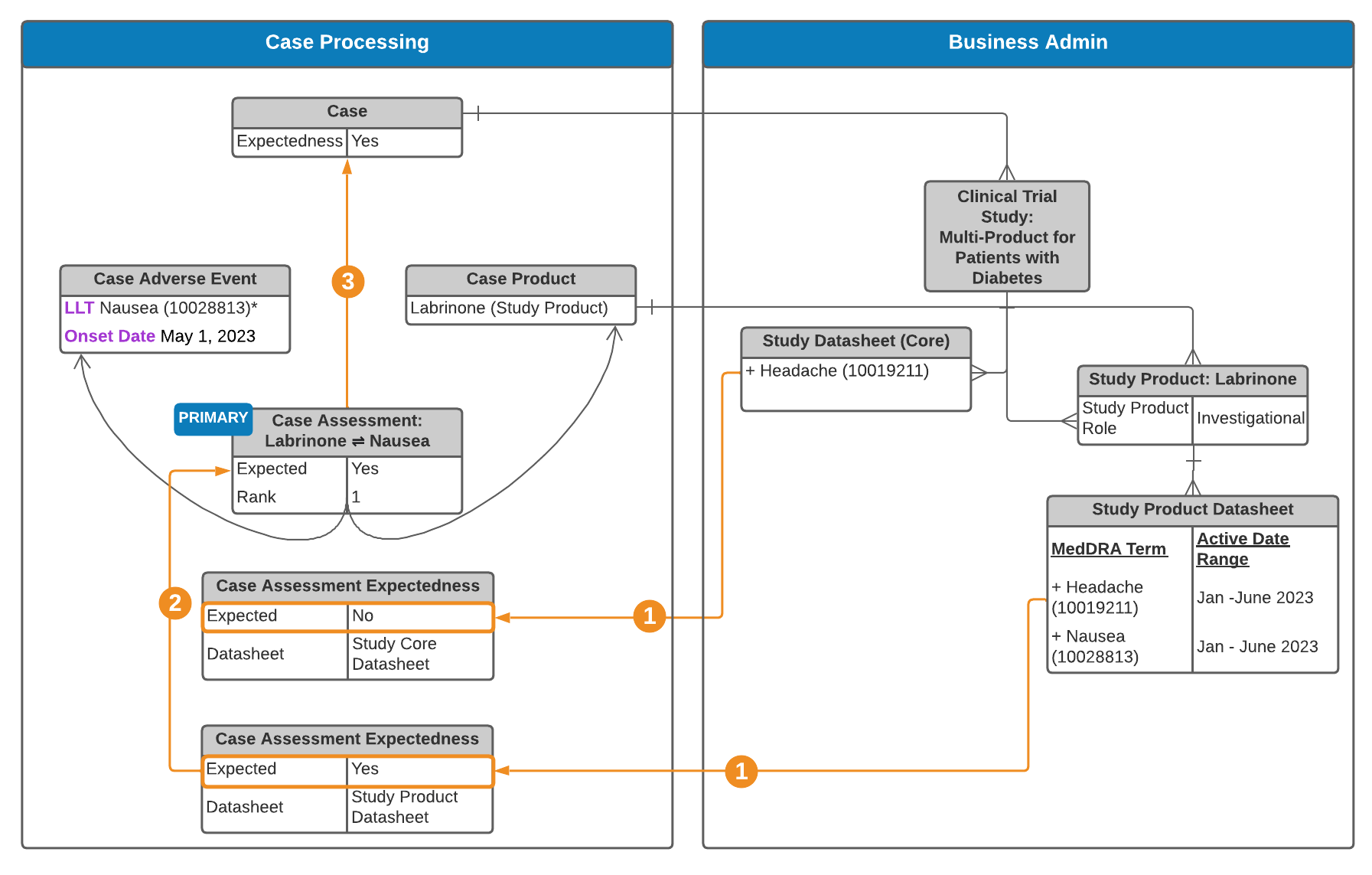
Multi-Product Clinical Trial Study Case
See an explanation of the steps below.
 Expectedness Generation
Expectedness Generation
Vault generates Case Assessment Expectedness records for each relevant Datasheet:
- Non-Study Cases: For each product Datasheet (product family, product core, or local) related to Case Products.
- Study Cases: Based on the following Datasheets, when configured:
- Study product
- Study core
- Product (product family, product core, or local) related to Case Products
Note: When both Study Product Datasheets and Study Core Datasheets are set up, Case Assessment Expectedness records are generated for both. If your Admin has enabled Agency-Based Auto-Expectedness for Clinical Trial Study Cases or Datasheet Expectedness for Blinded Study Products, see Expectedness Evaluations for Clinical Trial Study Cases for how Vault may generate additional Case Assessment Expectedness records.
 Case Assessment Listedness and Expectedness
Case Assessment Listedness and Expectedness
To automatically populate the Expected and Listedness fields on a Case Assessment, Vault looks at the Case Assessment’s Expectedness records using the following logic:
To populate the Listedness field on the Case Assessment:
| Case Report Type | Auto-Population Logic |
|---|---|
| Non-Study Case | If there is an Expectedness record corresponding to the Product's Core or Product Family Datasheet, this rolls up to populate the Listedness field on the Case Assessment. If there are no Expectedness records, the Listedness field is not automatically populated. |
| Study Case | If there is an Expectedness record corresponding to the Study Core Datasheet, this Expectedness rolls up to populate the Listedness field on the Case Assessment. If there is no Study Product or Study Core Datasheet, but there is an Expectedness record corresponding to the Product's Core or Product Family Datasheet, this value rolls up to the Listedness field. If there are no Expectedness records, the Listedness field is not automatically populated. |
To populate the Expected field on the Case Assessment:
| Case Report Type | Auto-Population Logic |
|---|---|
| Non-Study Case | Vault evaluates all Expectedness records under the Case Assessment to set the Expected field, using the following logic:
|
| Study Case | If there are Expectedness records, Vault roll-up for Study Products is as follows:
If there is no Study Product or Study Core Datasheet, Vault evaluates all Expectedness records under the Case Assessment to set the Expected field as follows:
|
 Case Listedness and Expectedness
Case Listedness and Expectedness
At the Case-level, for both study and non-study Cases, both the Listedness (Core) and Case Expectedness fields are synced with the primary Case Assessment. Unless the Case fields are overridden, any time the primary Case Assessment changes, Vault updates the associated Case-level fields to match.
Study Product Placeholder Expectedness Roll-Up Evaluation
If your Admin has enabled Datasheet Expectedness for Blinded Study Products, for double-blinded clinical trial study Cases without Study Arms, Vault generates Case Assessment, Case Assessment Result, and Expectedness records based on each Datasheet associated with Study Product Placeholders on the blinded Case. Vault rolls up the most conservative Expected value across all Expectedness records, including overridden values, to the Expected field of the associated Case Assessment. For example, if there are multiple Expectedness records and only one (1) has Expected set to No, Vault rolls up No to the Case Assessment regardless of the Datasheet type.
Override Case Expectedness or Listedness
There are two ways to update the Case Expectedness or Listedness fields:
- Edit the field on the primary Case Assessment to override the calculated expectedness. The associated (Status) field updates to “Overridden” on the Case Assessment but remains Auto Calculated on the Case.
This will automatically update the associated field at the Case-level, because the Case fields are synced with the primary Case Assessment.
Note: Overridden Case Assessment Expectedness records are not deleted or re-created, unless an associated Product or Adverse Event is updated.
- Edit the field directly on the Case to override the value, and prevent Vault from syncing the value with the primary Case Assessment. The associated (Status) field updates to “Overridden” on the Case.
Note: If you use this option, Vault will not update the Case Expectedness or Listedness to match any future changes to the associated fields on the primary Case Assessment.
How-To Video: Overriding Auto-Expectedness Evaluations
Watch our quick video tutorial on Overriding Auto-Expectedness Evaluations.