Safety Workbench provides U.S. Periodic Adverse Drug Experience Report (PADER) authoring and table generation capabilities. All generated Workbench PADER aggregate reports are unmasked.
The following table summarizes the PADER tabulations that Safety Workbench generates:
| Tabulation | Description |
|---|---|
| 15-Day and Non-15 Day Summary Reports | This report prints the number of Case Adverse Events that match report criteria. Vault generates tables for both initial reports and follow-up reports. |
| 15-Day Report Line Listing Summary | This report is a summary listing of Cases that are reportable within 15 days, broken down by initial/follow-up. |
| Non-Expedited Line Listing Summary | This report is a summary listing of non-expedited Cases and Submissions broken down by initial/follow-up. |
| Summary of ADR from Postmarketing Sources | This report prints the number of Case Adverse Events that match report criteria. |
| Interval Line Listings | This report prints the Case listing that matches report criteria. Vault categorizes Case listings by initial and follow-up reports, seriousness, listedness, and whether the report was expedited. |
| Cases Submitted Under Other NDAs | This report prints the Case listing that matches report criteria. This report is a summary listing of the Adverse Events in which the drug or biological Product was listed as one of the suspect Products, but the report was filed to another New Drug Application (NDA), Abbreviated New Drug Application (ANDA), or Biologic License Application (BLA) held by the applicant. |
| Malfunction Line Listing | For Cases that contain combination products with a Device product type that has malfunctioned, this report prints a listing of device constituent malfunctions. |
| Line Listing Summary for Combination Products | This report is a summary listing of Cases that contain combination products with a Device product type that has malfunctioned. |
| Malfunction Tabulation | For Cases that contain combination products with a Device product type that has malfunctioned, this report prints the number of Case Adverse Events that match report criteria. |
| List of Death Cases | This report lists all the Cases that were transmitted to the FDA during the reporting period as either a 15-day or a non-15-day report, where the patient has died. |
| List of Nullified Cases | This report is a listing of nullified Cases during the reporting interval. |
| Case Series | This report is a listing of all Cases on the PADER, provided in a flattened Excel format. |
Note: Your Admin can configure custom Workbench PADER report templates for your organization.
Safety Workbench allows you to generate, run, and download multiple Workbench aggregate reports at once. If this option is relevant to your organization’s business processes, see Generate & Run Report Sets.
Prerequisites
To generate Workbench PADERs, consider the following prerequisites:
- Your Admin must configure Workbench PADER aggregate reports.
- Your Admin must grant you permissions to view and prepare aggregate reports. Typically, these permissions are reserved for the Aggregate Report Writer role.
- Your Admin must configure US-based local product Datasheets that list expected adverse events for the reporting family Product. Workbench uses product Datasheets to classify adverse events as Labeled or Unlabeled in Workbench PADER tables.
- To generate table data from Study-type Cases, your Admin must configure the appropriate Study Products.
- A Case must have a Transmission record with a Transmission Date.
Note: Workbench generates Workbench PADER tables using controlled vocabularies configured by your Admin to determine when causality is not related.
Create a Workbench PADER
Create a Workbench PADER aggregate report and specify the report parameters.
Add a Workbench PADER Report Set
To create a Workbench PADER:
- Navigate to Workbench > Report Sets.
- Select Create.
- On the Create Workbench Report Set page, enter a name for the report.
- Select Save or Save + Create to save the Workbench Report Set and create another.
- Add Workbench Report Definitions in the Reports to Generate section and filters in the Filters section. Then, see the section below for completing the required filters for the Workbench PADER.
When you create a Workbench Report Set, Vault sets the lifecycle state to Draft.
Specify Workbench PADER Filters
After adding Workbench Report Definitions to the Workbench PADER report set and selecting Refresh in the Filters section, required filters appear in the Filters section. Specify values for the following filters:
- Aggregate Reporting Group: Select an Aggregate Reporting Group configured for aggregate reporting from the drop-down or use the Advanced Search (
 ) icon to use filters and refine your search. To learn more about Aggregate Reporting Groups, see Configure Workbench PADER Aggregate Reports.
) icon to use filters and refine your search. To learn more about Aggregate Reporting Groups, see Configure Workbench PADER Aggregate Reports. - Transmission > Generation Date: Enter the start and end date for the reporting period. Vault uses the Cases within the reporting period to generate table data. Cases are included when the Case Transmission is within the reporting period.
Generate and Run Workbench PADER Reports
Review and verify the Workbench PADER report set. Once you have confirmed the report set details are correct, see Generate & Run Report Sets for information on generating and running reports.
Workbench PADER Table Generation Data Mapping
Workbench populates aggregate report tables using Cases within the reporting period specified on the Workbench PADER and the product registrations configured on the associated Aggregate Reporting Group.
The following sections describe how Workbench generates PADER tabulations:
- 15-Day and Non-15-Day Summary Reports
- 15-Day Report Line Listing Summary
- Non-Expedited Line Listing Summary
- Summary of ADR from Postmarketing Sources
- Interval Line Listings
- Cases Submitted Under Other NDAs
- Malfunction Line Listing
- Line Listing Summary for Combination Products
- Malfunction Tabulation
- List of Death Cases
- List of Nullified Cases
- Case Series
15-Day and Non-15-Day Summary Reports
Case-Based Report: This report prints the number of Cases that match report criteria, using the primary Case Adverse Event to categorize the Case into the appropriate column.
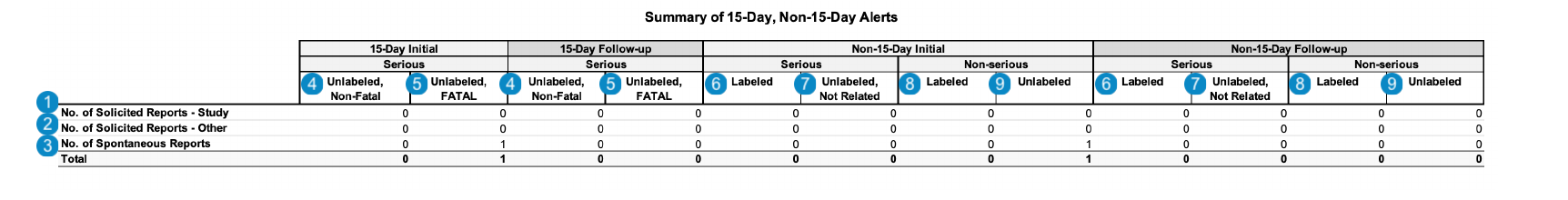
Vault generates the 15-Day and Non-15-Day Summary Reports table for both initial and follow-up reports. The following image displays the Summary of 15-Day and Non-15-Day Alerts table, which includes both initial and follow-up reports:
Table Constraints
Vault filters Cases to include in the Workbench PADER 15-Day and Non-15-Day Summary Reports using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault finds Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The registration on the Transmission record matches that listed in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
A Case Product must meet the following conditions:
- The Product Registration field must link to a Product Registration record added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Drug Role field must be set to Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one (1) FDA Transmission within the reporting period with the Transmission Reason set to one (1) of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is set to
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one (1) of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not set to
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
Sorting: Cases are sorted in ascending order by Worldwide UID (WWUID).
| Number | Name | Description | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Solicited Reports - Study | The number of Cases that match one (1) of the following scenarios:
COUNT IF
|
|||||||||||||
| No. of Solicited Reports - Other | The number of Cases that match one (1) of the following scenarios:
COUNT IF
|
|||||||||||||
| No. of Spontaneous Reports | The number of Cases with the Case > Report Type set to one (1) of the following:
|
|||||||||||||
| Serious, Unlabeled, Non-Fatal (initial and follow-up) | The sum of Cases with primary adverse events that meet the following criteria:
|
|||||||||||||
| Serious, Unlabeled, Fatal (initial and follow-up) | The sum of Cases with primary adverse events that meet the following criteria:
|
|||||||||||||
| Serious, Labeled (initial and follow-up) | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
|||||||||||||
| Serious, Unlabeled, Not Related (initial and follow-up) | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
|||||||||||||
| Non Serious, Labeled (initial and follow-up) | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
|||||||||||||
| Non Serious, Unlabeled (initial and follow-up) | The sum of Cases with primary Case Adverse Events that meet the following criteria:
|
15-Day Report Line Listing Summary
Submission-Based Report: This report prints the number of Case submissions that match report criteria.
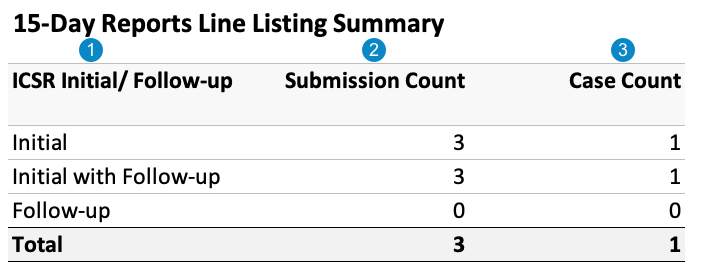
The following image displays the 15-Day Report Line Listing Summary table:
Table Constraints
Vault filters Cases to include in the PADER 15-Day Report Line Listing Summary using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The Reportable Product Registration on the Transmission matches that in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
If the PADER is run for a non-combination product:
A Case Product must meet the following conditions:
- The Product Registration field of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
If the PADER is run for a combination product, a different registration field is considered:
A Case Product must meet the following conditions:
- The Combination Product Registration of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Combination Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one FDA Transmission within the reporting period with the Transmission Reason set to one of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
| Number | Name | Description |
|---|---|---|
| ICSR Initial/ Follow-up |
Cases in the Initial category include an initial Transmission in the report interval. Cases in the Initial with Follow-up category include an initial Transmission and at least one follow-up Transmission in the report interval. This is a subset of the Initial category. Cases in the Follow-up category do not include any initial Transmissions in the report interval. |
|
| Submission Count |
For the Initial, Initial with Follow-up, and Follow-up categories, Vault counts the number of Submissions for Cases in each category. |
|
| Case Count | For the Initial, Initial with Follow-up, and Follow-up categories, Vault counts the number of Cases in each category. |
Non-Expedited Line Listing Summary
Submission-Based Report: This report prints the number of non-expedited Case submissions that match the report criteria.
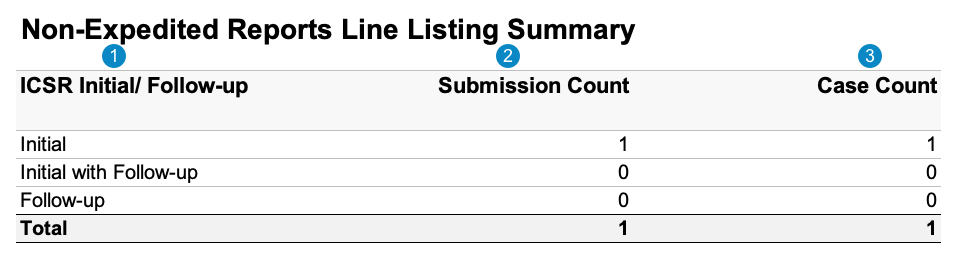
The following image displays the Non-Expedited Line Listing Summary table:
Table Constraints
Vault filters Cases to include in the PADER Non-Expedited Line Listing Summary using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The Reportable Product Registration on the Transmission matches that in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
If the PADER is run for a non-combination product:
A Case Product must meet the following conditions:
- The Product Registration field of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
If the PADER is run for a combination product a different registration field is considered:
A Case Product must meet the following conditions:
- The Combination Product Registration of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Combination Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one FDA Transmission within the reporting period with the Transmission Reason set to one of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
| Number | Name | Description |
|---|---|---|
| ICSR Initial/ Follow-up |
Cases in the Initial category include an initial Transmission in the report interval. Cases in the Initial with Follow-up category include an initial Transmission and at least one follow-up Transmission in the report interval. This is a subset of the Initial category. Cases in the Follow-up category do not include any initial Transmissions in the report interval. |
|
| Submission Count |
For the Initial, Initial with Follow-up, and Follow-up categories, Vault counts the number of Submissions for Cases in each category. |
|
| Case Count | For the Initial, Initial with Follow-up, and Follow-up categories, Vault counts the number of Cases in each category. |
Summary of ADR from Postmarketing Sources
Event-Based Report: This report prints the number of Case Adverse Events that match report criteria.
Note: For the Workbench PADER Summary of ADR from Postmarketing Sources report output, if a Case has multiple LLT Adverse Events that fall under the same PT, Vault counts the PT only once.
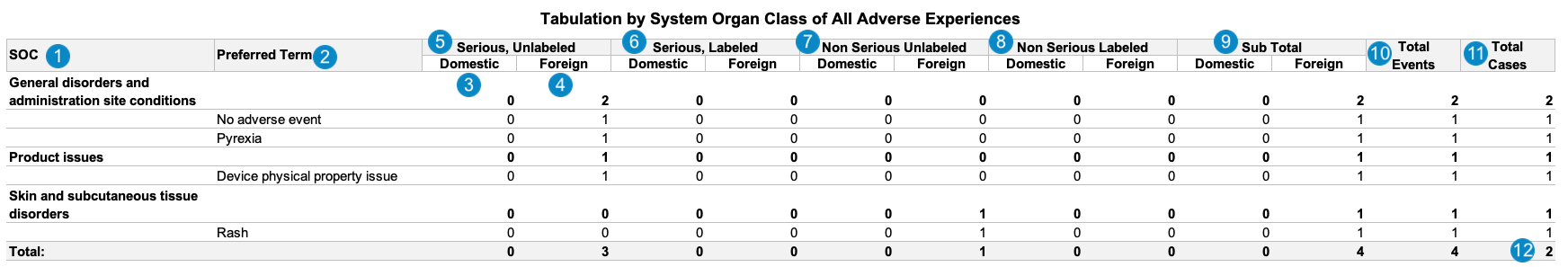
The following image displays the Workbench PADER Summary of ADR from Postmarketing Sources report:
Table Constraints
Vault filters Cases to include in the Workbench PADER Summary of Adverse Drug Reactions from Postmarketing Sources using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault finds Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The registration on the Transmission record matches that listed in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
A Case Product must meet the following conditions:
- The Product Registration field must link to a Product Registration record added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Drug Role field must be set to Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Note: The Workbench PADER Summary of ADR from Postmarketing Sources report considers only Case Adverse Events with Case Assessments.
Table Mapping
Sorting: Cases are sorted in ascending order, first by UID and then by Worldwide UID.
| Number | Name | Description |
|---|---|---|
| SOC | The MedDRA System Organ Class (SOC) for the Case Adverse Event. The sum of all adverse events under this SOC is listed under each column.event_meddra__v.soc_term__v
|
|
| Preferred Term | The MedDRA Preferred Term (PT) coded on the Case Adverse Event, grouped by the MedDRA SOC.case_adverse_event__v.event_meddra__v.pt_term__v
|
|
| Domestic | The sum of adverse events where the Case Adverse Event > Event Country field is set to a Country where the Agency field is set to FDA.case_adverse_event__v.event_country__v.agency__v = fda__v
|
|
| Foreign | The sum of adverse events where the Case Adverse Event > Event Country field is set to a Country where the Agency field is not set to FDA.case_adverse_event__v.event_country__v.agency__v ≠ fda__v
|
|
| Serious, Unlabeled | Primary adverse events that meet the following criteria:
|
|
| Serious, Labeled | Primary adverse events that meet the following criteria:
|
|
| Non Serious Unlabeled | Primary adverse events that meet the following criteria:
|
|
| Non Serious Labeled | Primary adverse events that meet the following criteria:
|
|
| Subtotal | The subtotal sum of adverse events for each MedDRA SOC and PT, within domestic or foreign jurisdiction. | |
| Total Events | The total sum of domestic and foreign adverse events for each MedDRA SOC and PT. | |
| Total Cases | The total sum of Cases for each MedDRA SOC and PT. | |
| Grand Total | The total sum of all adverse events and Cases. |
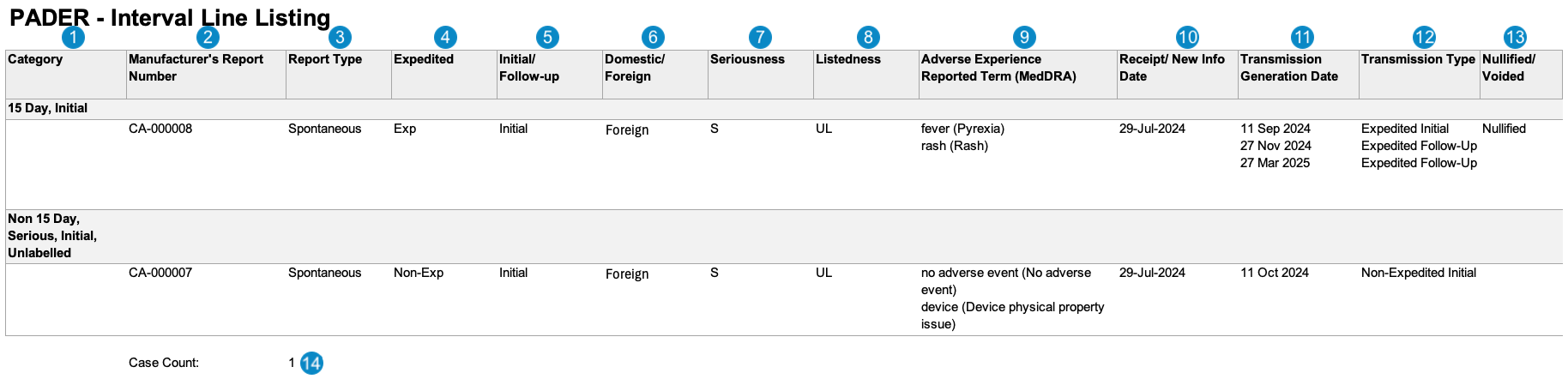
Interval Line Listings
Case-Based Report: This report prints the number of Cases that match report criteria.
Workbench generates Interval Line Listing tables for both initial and follow-up reports.
The following image displays the Workbench PADER Interval Line Listing, which includes both initial and follow-up reports.
Table Constraints
Vault filters Cases to include in the Workbench PADER Interval Line Listings using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault finds Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The registration on the Transmission record matches that listed in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
A Case Product must meet the following conditions:
- The Product Registration field must link to a Product Registration record added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Drug Role field must be set to Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one (1) FDA Transmission within the reporting period with the Transmission Reason set to one (1) of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is set to
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one (1) of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not set to
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
For each Case included in the report, the Workbench PADER Interval Line Listings displays all Case Adverse Events.
Table Mapping
| Number | Name | Description |
|---|---|---|
| Category |
Vault groups Cases based on the following Case criteria:
|
|
| Manufacturer's Report Number | Vault maps this value from the Case > UID field.case_version__v.uid__v
|
|
| Report Type | The Report Type of the Case.case_version__v.report_type__v
|
|
| Expedited |
Whether the Case was expedited. Vault populates Vault populates |
|
| Initial/Follow-up |
Whether the Case is an initial or follow-up Case. Vault populates Otherwise, Vault populates |
|
| Domestic/Foreign |
Whether the Case is domestic or foreign. Vault populates Vault populates |
|
| Seriousness |
Whether the Case is serious or non-serious. Vault populates Otherwise, Vault populates |
|
| Listedness |
Whether the Case is listed or unlisted. Vault populates
(case_assessment__v.case_assessment_expectedness__v.expected__v
Otherwise, Vault populates |
|
| Adverse Experience Reported Term (MedDRA) | Both the reported name and the MedDRA Preferred Term (PT) for the adverse events. Vault orders the adverse events by rank, blank values ranking last. |
|
| Receipt/New Info Date | Vault maps this value from the New Info Date field on the Case. If the New Info Date is blank, Vault maps this value from the Case Receipt date. |
|
| Transmission Generation Date | Vault populates this field with all Transmission Dates for Case Transmissions that meet the following criteria:
|
|
| Transmission Type | Vault determines the Transmission Type using the Transmission Reason and Local Expedited Criteria fields on Transmissions associated with the Case. For each Transmission date listed in the Transmission Generation Date column, Vault displays one (1) of the following:
Vault determines a Case is expedited if one (1) or more FDA Transmissions within the reporting period have the Local Expedited Criteria field set to Yes or blank. Vault determines a Case is an initial Case if at least one (1) FDA Transmission within the reporting period has the Transmission Reason set to one (1) of the following:
Otherwise, Vault determines the Case is a follow-up Case if all FDA Transmissions within the reporting period have the Transmission Reason set to one (1) of the following:
|
|
| Nullified/Voided |
Vault populates Otherwise, Vault leaves this value blank. |
|
| Case Count |
Vault displays the total number of Cases on the report. |
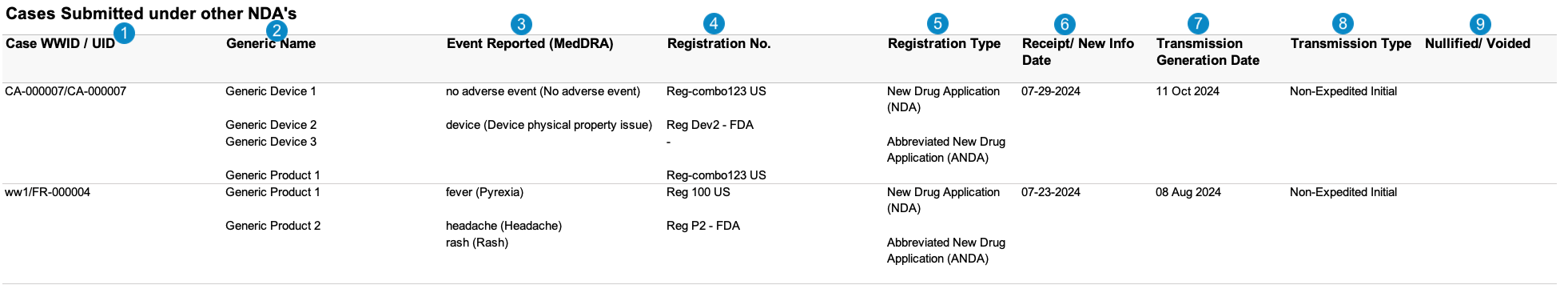
Cases Submitted Under Other NDAs
Case-Based Report: This report lists the Cases that match report criteria.
The Cases Submitted Under Other NDAs report is a Case line listing in which the drug or biological Product was listed as one (1) of the suspect Products on the Case, but the Case was submitted to the FDA under another New Drug Application (NDA), Abbreviated New Drug Application (ANDA), or Biologic License Application (BLA) held by the applicant.
If at least one (1) reportable Product Registration on the FDA Transmission for the Case matches that of the Aggregate Reporting Group, Vault includes this Case in one (1) of the following reports:
- 15-Day and Non-15-Day Summary Reports
- Summary of ADR from Postmarketing Sources
- Interval Line Listings
If the reportable Product Registration on the FDA Transmission for the Case does not match that of an Aggregate Reporting Group, Vault includes the Case in the Cases Submitted Under Other NDAs report. For more information about how Vault identifies Cases as submitted under another NDA, ANDA, or BLA, see Configure Workbench PADER Aggregate Reports.
Table Constraints
Vault filters Cases to include in the Cases Submitted Under Other NDAs report using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault finds Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The registration on the Transmission record does not match that listed in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v != aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
A Case Product must meet the following conditions:
- The Product Registration field must link to a Product Registration record added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Drug Role field must be set to Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Table Mapping
Sorting: Cases are sorted in ascending order, first by UID and then by Worldwide UID.
| Number | Name | Description |
|---|---|---|
| Case WWID/UID | Vault maps these values from the following fields:
|
|
| Generic Name | The name of each suspect or interacting Case Product.case_version__v.case_product__v.product_name__v
|
|
| Event Reported (MedDRA) | The MedDRA PT of each Case Adverse Event, sorted by the primary event first.case_adverse_event__v.event_meddra__v.pt_term__v
|
|
| Registration No. | The value from the Registration Number field on the Product Registration associated with the Case Product.case_product__v.product_registration__v.registration_number__v
|
|
| Registration Type | The value from the Registration Type field on the Product Registration associated with the Case Product.case_product__v.product_registration__v.registration_type__v
|
|
| Receipt/New Info Date | Vault maps this value from the New Info Date field on the Case. If the New Info Date is blank, Vault maps this value from the Case Receipt date.IF not blank(case_version__v.new_info_idate__v)
|
|
| Transmission Generation Date | Vault populates this field with the most recent Transmission Date for a Case Transmission that meets the following criteria:
|
|
| Transmission Type | Vault determines the Transmission Type using the Transmission Reason and Local Expedited Criteria fields on Transmissions associated with the Case. Vault populates this field with one (1) of the following values:
Vault determines a Case is expedited if one (1) or more FDA Transmissions within the reporting period have the Local Expedited Criteria field set to Yes or blank. Vault determines a Case is an initial Case if at least one (1) FDA Transmission within the reporting period has the Transmission Reason set to one (1) of the following:
Otherwise, Vault determines the Case is a follow-up Case if all FDA Transmissions within the reporting period have the Transmission Reason set to one (1) of the following:
|
|
| Nullified/Voided |
Vault populates Otherwise, Vault leaves this value blank. |
Combination Product Reports
Workbench PADERs include Cases with combination products. If the combination product contains a device constituent that has malfunctioned and the combination product is in the Aggregate Reporting Group and on the FDA Transmission, the Case is eligible for the following PADERs:
See Manage Combination Products for more information about combination products and their constituents.
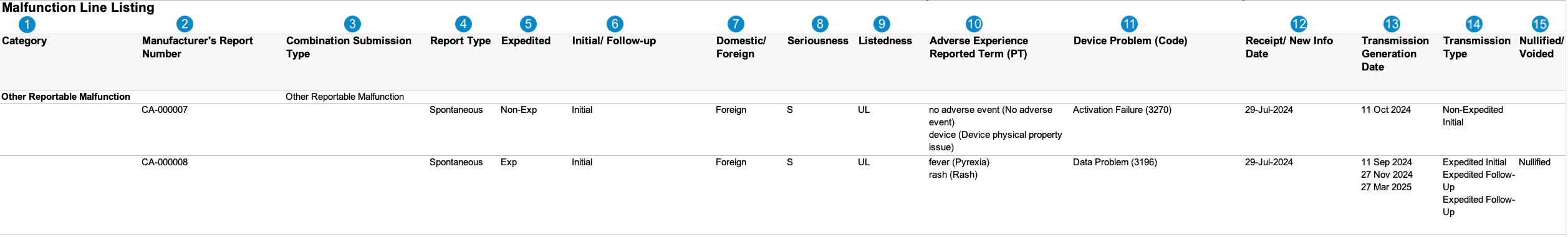
Malfunction Line Listing
Case-Based Report: This report lists the Cases that match report criteria.
The following image displays the Malfunction Line Listing table:
Table Constraints
Vault filters Cases to include in the PADER Malfunction Line Listing using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The registration on the Transmission record matches that listed in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
A Case Product must meet the following conditions:
- The Combination Product Registration field of a combination product must link to a Product Registration record added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Combination Product Registration field of a combination product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The combination product contains at least one (1) device constituent, and the device constituent has a malfunction (Malfunction is set to Yes).
Case_version__vr.case_product__v.malfunction__v = Yes WHERE Case_version__vr.case_product__v.product_type__v = Device - The Drug Role field must be set to Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one (1) FDA Transmission within the reporting period with the Transmission Reason set to one (1) of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is set to
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one (1) of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not set to
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
Sorting: Cases are sorted in ascending order, first by Combination Submission Type and then by Manufacturer’s Report Number.
| Number | Name | Description |
|---|---|---|
| Category |
Vault groups Cases based on the following Case criteria:
|
|
| Manufacturer's Report Number | Vault maps this value from the Case > UID field.case_version__v.uid__v
|
|
| Combination Submission Type | Vault maps this value from the Transmission > FDA Report Type. If the FDA Report Type is 5-Day, Vault sets the value to Otherwise, Vault sets the value to |
|
| Report Type | The Report Type of the Case.case_version__v.report_type__v
|
|
| Expedited |
Whether the Case was expedited. Vault populates Vault populates |
|
| Initial/Follow-up |
Whether the Case is an initial or follow-up Case. Vault populates Otherwise, Vault populates |
|
| Domestic/Foreign |
Whether the Case is domestic or foreign. Vault populates Vault populates |
|
| Seriousness |
Whether the Case is serious or non-serious. Vault populates Otherwise, Vault populates |
|
| Listedness |
Whether the Case is listed or unlisted. Vault populates
(case_assessment__v.case_assessment_expectedness__v.expected__v
Otherwise, Vault populates |
|
| Adverse Experience Reported Term (PT) |
Both the reported name and the MedDRA Preferred Term (PT) for the adverse events. |
|
| Device Problem (Code) | The FDA Device Code: Case product > Device Problem > Device Code> Level x Code, where "x" is the product term level (either 1, 2, or 3). Vault sets the value using the following logic:
|
|
| Receipt/New Info Date | Vault maps this value from the New Info Date field on the Case. If the New Info Date is blank, Vault maps this value from the Case Receipt date. |
|
| Transmission Generation Date | Vault populates this field with all Transmission Dates for Case Transmissions that meet the following criteria:
|
|
| Transmission Type | Vault determines the Transmission Type using the Transmission Reason and Local Expedited Criteria fields on Transmissions associated with the Case. For each Transmission date listed in the Transmission Generation Date column, Vault displays one (1) of the following:
Vault determines a Case is expedited if one (1) or more FDA Transmissions within the reporting period have the Local Expedited Criteria field set to Yes or blank. Vault determines a Case is an initial Case if at least one (1) FDA Transmission within the reporting period has the Transmission Reason set to one (1) of the following:
Otherwise, Vault determines the Case is a follow-up Case if all FDA Transmissions within the reporting period have the Transmission Reason set to one (1) of the following:
|
|
| Nullified/Voided |
Vault populates Otherwise, Vault leaves this value blank. |
How Vault Determines Expectedness
Vault sets the Expectedness field on a Case. To determine whether an Adverse Event is expected for combination products with a device constituent, Vault looks at the non-device constituent and finds all Expectedness records on Case Assessments that meet the following criteria:
- Is attached to a Registration record of a combination product.
- Lists a country that is supported by the FDA.
- Has a datasheet registration that matches the registration in the Aggregate Reporting Group.
If at least one (1) record has the Expected field set to No or blank, Vault determines an Adverse Event is unexpected.
How Vault Determines Listedness
Vault sets the Listedness field on a Case. For combination products with a device constituent, Vault determines an Adverse Event is listed if the non-device constituent in the Aggregate Reporting Group is associated with an unexpected Adverse Event.
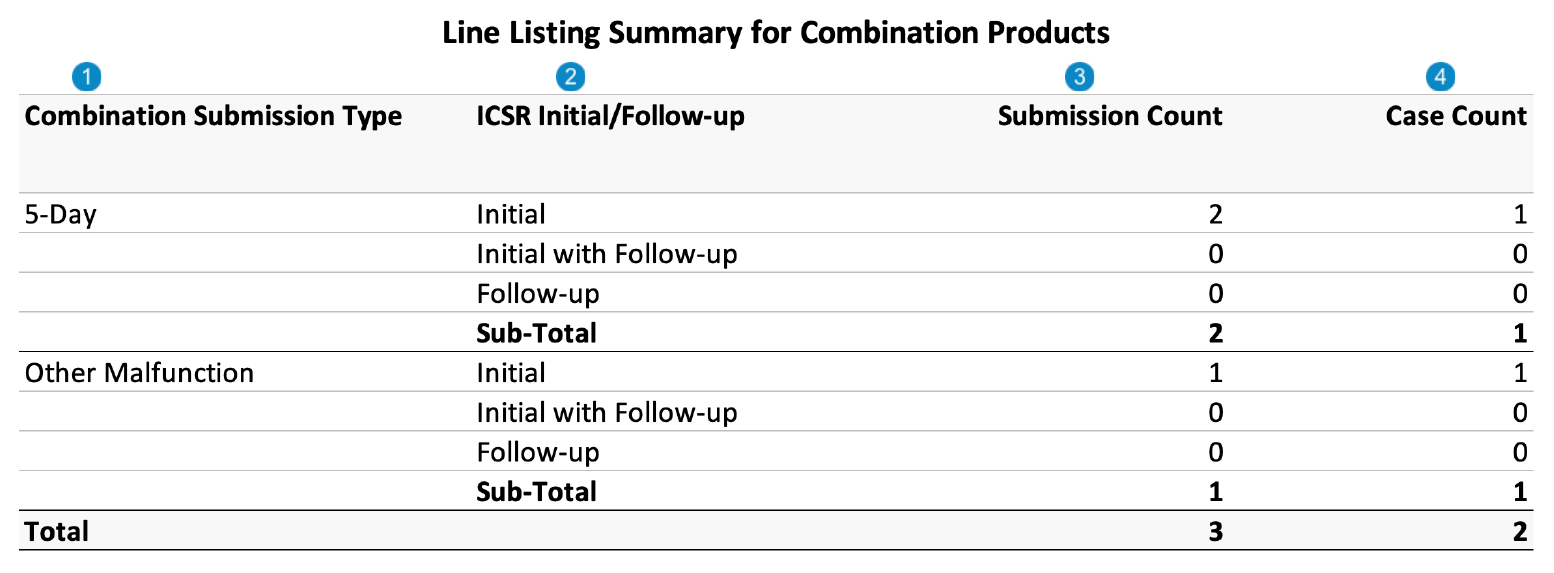
Line Listing Summary for Combination Products
Case-Based Report: This report lists the Cases that match report criteria.
The Case count in this report will match the Malfunction Line Listing report.
The following image displays the Line Listing Summary for Combination Products table:
Table Constraints
Vault filters Cases to include in the PADER Line Listing Summary for Combination Products using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The registration on the Transmission record matches that listed in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
A Case Product must meet the following conditions:
- The Product Registration field of a combination product must link to a Product Registration record added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Product Registration field of a combination product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The combination product contains at least one (1) device constituent, and the device constituent has a malfunction (Malfunction is set to Yes).
Case_version__vr.case_product__v.malfunction__v = Yes WHERE Case_version__vr.case_product__v.product_type__v = Device - The Drug Role field must be set to Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one (1) FDA Transmission within the reporting period with the Transmission Reason set to one (1) of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is set to
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one (1) of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not set to
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
| Number | Name | Description |
|---|---|---|
| Combination Submission Type | Vault maps this value from the Transmission > FDA Report Type. If the FDA Report Type is 5-Day, Vault sets the value to Otherwise, Vault sets the value to |
|
| ICSR Initial/Follow-up | Cases in the Initial category include an initial Transmission in the report interval. Cases in the Initial with Follow-up category include an initial Transmission and at least one (1) follow-up Transmission in the report interval. This is a subset of the Initial category. Cases in the Follow-up category do not include any initial Transmissions in the report interval. |
|
| Submission Count | For the Initial, Initial with Follow-up, and Follow-up categories, Vault counts the number of Submissions for Cases in each category. Note: Submission counts align with the number of Transmissions listed on the Malfunction Line Listing. |
|
| Case Count | For the Initial, Initial with Follow-up, and Follow-up categories, Vault counts the number of Cases in each category. |
Malfunction Tabulation
Event-Based Report: This report prints the number of Case Adverse Events that match report criteria.
The following image displays the Malfunction Tabulation:
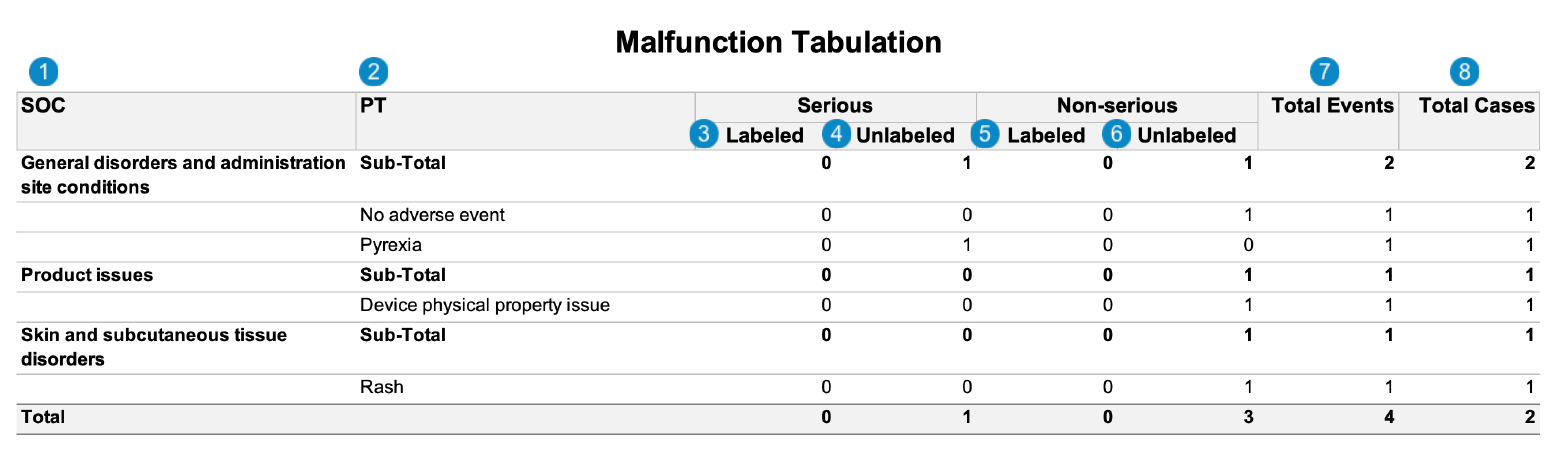
Table Constraints
Vault filters Cases to include in the PADER Malfunction Tabulation using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The registration on the Transmission record matches that listed in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
A Case Product must meet the following conditions:
- The Product Registration field of a combination product must link to a Product Registration record added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registrations__v.registration_number__v - The Product Registration field of a combination product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The combination product contains at least one (1) device constituent, and the device constituent has a malfunction (Malfunction is set to Yes).
Case_version__vr.case_product__v.malfunction__v = Yes WHERE Case_version__vr.case_product__v.product_type__v = Device - The Drug Role field must be set to Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Table Mapping
Sorting: Cases are sorted in ascending order, first by SOC and then by PT.
List of Death Cases
Case-Based Report: This report lists the Cases that match report criteria sorted by WWID/UID.
The following image displays the List of Death Cases table:
Table Constraints
Vault filters Cases to include in the PADER List of Death Cases using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The Reportable Product Registration on the Transmission matches that in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
If the PADER is run for a non-combination product:
A Case Product must meet the following conditions:
- The Product Registration field of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registrations__v - The Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
If the PADER is run for a combination product a different registration field is considered:
A Case Product must meet the following conditions:
- The Combination Product Registration of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registrations__v - The Combination Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one FDA Transmission within the reporting period with the Transmission Reason set to one of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
| Number | Name | Description |
|---|---|---|
| Case WWID/ UID | Vault maps these values from the following fields:
|
|
| Country Gender Age |
Vault maps values from the following fields:
|
|
| Suspect Drug |
Vault maps the Product Names of all Case Products on the Case whose Drug Role is set to Suspect, Interacting, or Drug not Administered, ordered by rank.
|
|
| Adverse Events |
Vault lists the MedDRA PT of all serious and non-serious adverse events on the Case. If the outcome of an adverse event is fatal, Vault appends the name of the outcome in parentheses. For example, |
|
| Cause of Death (PT) |
Both MedDRA PT and reported term entered on the Case > Cause of Death record.
|
|
| Autopsy Details |
The Autopsy value on the Case:
|
|
| Date of Death |
The Date of Death on the Case:
|
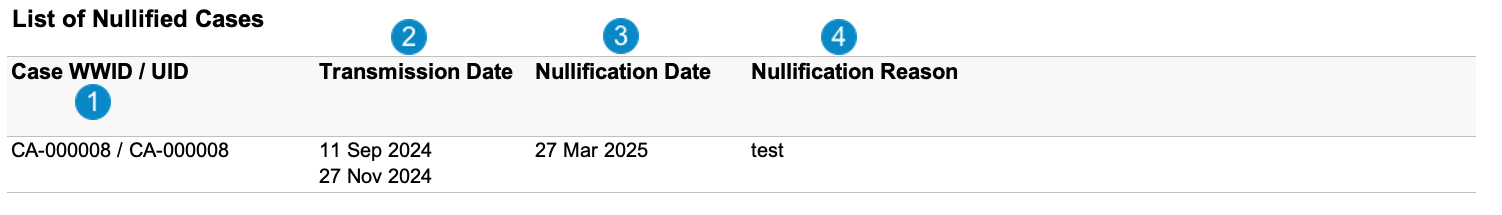
List of Nullified Cases
Case-Based Report: This report lists the Cases that match report criteria, sorted by WWID/UID.
The following image displays the List of Nullified Cases table:
Table Constraints
Vault filters Cases to include in the PADER List of Nullified Cases using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The Reportable Product Registration on the Transmission matches that in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
If the PADER is run for a non-combination product:
A Case Product must meet the following conditions:
- The Product Registration field of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registrations__v - The Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
If the PADER is run for a combination product a different registration field is considered:
A Case Product must meet the following conditions:
- The Combination Product Registration of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Combination Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one FDA Transmission within the reporting period with the Transmission Reason set to one of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
| Number | Name | Description |
|---|---|---|
| Case WWID/ UID | Vault maps these values from the following fields:
|
|
| Transmission Date |
The Transmission Date on the Transmission where the Reason on the Transmission is Initial, Follow-up, or Amendment.
|
|
| Nullification Date |
The Transmission Date on the Transmission where the Reason on the Transmission is Nullified.
|
|
| Nullification Reason |
The Reason Text on the Transmission where the Reason on the Transmission is Nullified:
|
Case Series
Case-Based Report: This report lists the Cases that match any of the report criteria in the PADER report set, sorted by Case Name.
The following image displays the Case Series table:
Table Constraints
Vault filters Cases to include in the PADER Case Series using the following constraints:
Case Not Suppressed
The Suppress Submission field on the Case must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
FDA Transmission in Reporting Period
Vault filters by the Transmission Date to find Cases submitted to the FDA within the reporting period.
Cases must have an associated Transmission that meets the following conditions:
- The Transmission Destination is FDA.
case_version__v.transmission__v.destination__v = fda__v - The Transmission Date is within the interval reporting period specified in the Workbench PADER report set.
transmission__v.transmission_date__v >= transmission_generation.filter_start_range AND transmission__v.transmission_date__v <= transmission_generation.filter_end_range - The Transmission is in the E2B ACK Accepted or Completed lifecycle state, and the Transmission lifecycle state type is not Deleted.
case_version__v.transmission__v.state__v = (e2b_ack_accepted_state__v OR completed_state__v) AND case_version__v.transmission__v.state__v.statetype__v != Deleted - The Reportable Product Registration on the Transmission matches that in the Aggregate Reporting Group.
case_version__v.transmission__v.reportable_product_registration__v.registration_number__v = aggregate_reporting_group__v.product_registration__v.registration_number__v
The report lists only the latest version of the Case transmitted to the FDA within the reporting period.
Suspect, Interacting, or Drug Not Administered Product in Aggregate Reporting Group
If the PADER is run for a non-combination product:
A Case Product must meet the following conditions:
- The Product Registration field of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
If the PADER is run for a combination product a different registration field is considered:
A Case Product must meet the following conditions:
- The Combination Product Registration of a Case Product must link to a Product Registration added as a member of the Aggregate Reporting Group.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v IN aggregate_reporting_group__vr.aggregate_reporting_group_join__vr.product_registration__v.registration_number__v - The Combination Product Registration of a Case Product is on the FDA Transmission.
case_version__vr.case_product__v.combination_product_registration__v.registration_number__v = transmission__v.reportable_product_registration__v.registration_number__v - The Drug Role must be Suspect (E2B code=
1), Interacting (E2B code=3), or Drug Not Administered (E2B code=4).
case_product__v.drug_role__v.controlled_vocabulary__v.e2b_code__v = 1 OR 3 OR 4
Initial vs. Follow-Up Report
To determine whether Cases should be listed in initial or follow-up reports, Vault evaluates the Transmission Reason (transmission_reason__v) field on FDA Transmissions within the reporting period using the following logic:
- Initial reports: When there is at least one FDA Transmission within the reporting period with the Transmission Reason set to one of the following:
- Initial
- A custom Transmission Reason Controlled Vocabulary where the E2B code is
I(initial)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v = I
- Follow-up reports: When all FDA Transmissions within the reporting period have the Transmission Reason set to one of the following:
- Amendment
- Follow-Up
- A custom Transmission Reason Controlled Vocabulary where the E2B code is not
I(initial) or1(nullification)
transmission__v.reason__v.controlled_vocabulary__v.e2b_code__v ≠ I OR 1
Note: If the Transmission Reason is blank, Vault evaluates the Case as a follow-up.
Table Mapping
| Number | Name | Description |
|---|---|---|
| Case ID | Vault maps these values from the following fields:
|
|
| Case Name |
The Name of the Case.
|
|
| Domestic/Foreign |
Whether the Case is domestic or foreign. Vault populates Vault populates |
|
| Expedited |
Whether the Case was expedited. Vault populates Vault populates |
|
| Initial/ Follow-up |
Whether the Case is an initial or follow-up Case. Vault populates Otherwise, Vault populates |
|
| Listedness |
Whether the Case is listed or unlisted. Vault populates
(case_assessment__v.case_assessment_expectedness__v.expected__v
Otherwise, Vault populates |
|
| Nullified/ Voided |
Vault populates Otherwise, Vault leaves this value blank. |
|
| Report Type | The Report Type of the Case.case_version__v.report_type__v
|
|
| Seriousness |
Whether the Case is serious or non-serious. Vault populates Otherwise, Vault populates |
How Vault Determines Listedness
Vault evaluates Listedness on Cases. Vault determines a Case Adverse Event is listed if the non-device constituent in the Aggregate Reporting Group is associated with an unexpected Case Adverse Event. To do this, Vault finds all Case Assessment Expectedness records that:
- Are attached to a Product Registration record of a Product.
- List a country that is supported by the FDA.
- Have a datasheet registration that matches the registration in the Aggregate Reporting Group.
If at least one record has the Expected field set to No or blank, Vault determines a Case Adverse Event is unlisted.
If no Case Assessment Expectedness records are found, then Vault uses the Expectedness value on the Case Assessment record to determine Listedness.