Learn about SAR aggregate reports in Workbench and how to generate them.
About Workbench SARs
Safety Workbench provides Serious Adverse Reactions (SAR) authoring and table generation capabilities. Masked and unmasked versions of all reports can be generated.
The following table summarizes the SAR tabulations that Safety Workbench generates:
| Tabulation | Description |
|---|---|
| SAR Line Listing | This report prints the Case listings for SAR occurrences that match report criteria. |
| SAR Tabulation by PT | This report prints the Case listings for SAR occurrences that match report criteria, organized by the MedDRA Preferred Term (PT). |
| SAR Tabulation by SOC, PT | This report prints the Case listings for SAR occurrences that match report criteria, organized by the MedDRA System Organ Class (SOC) and then by the Preferred Term (PT). |
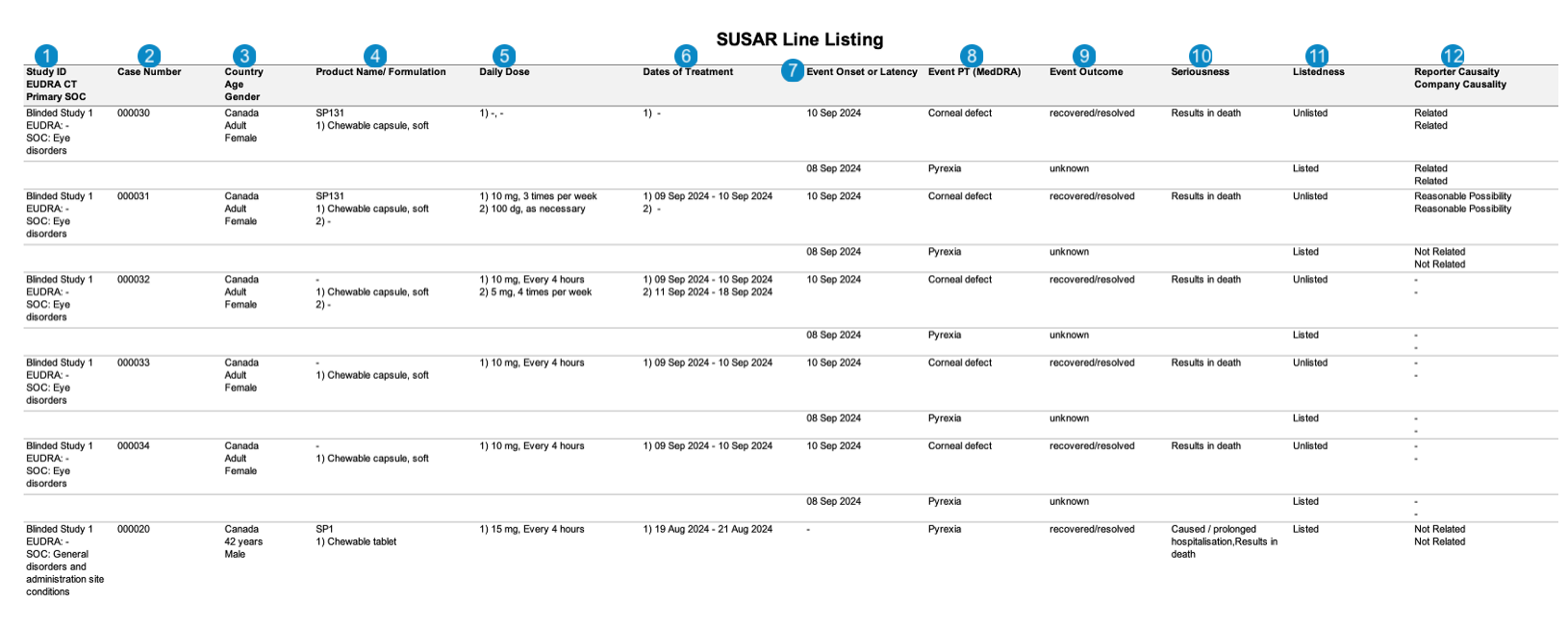
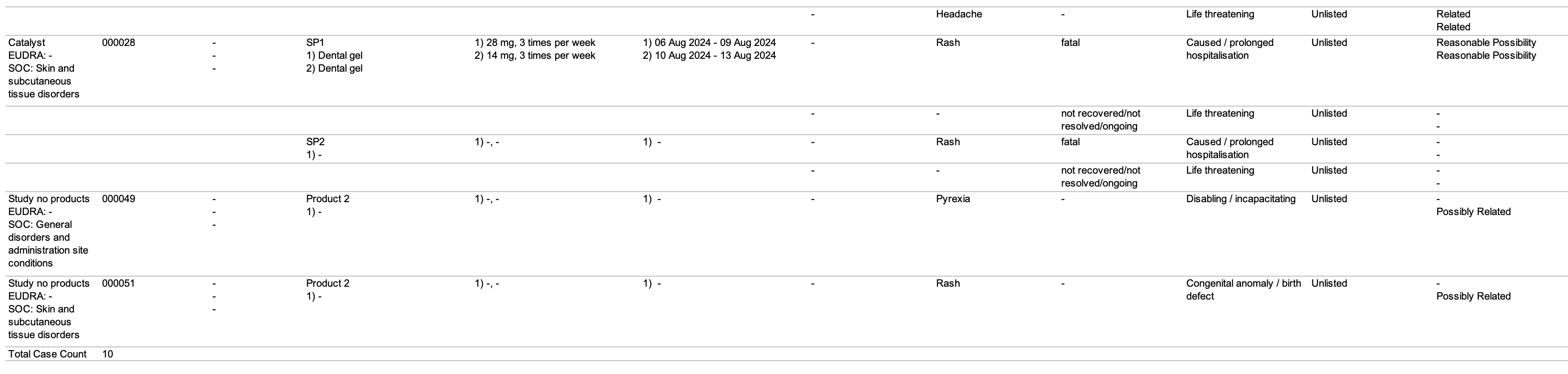
| SUSAR Line Listing | This report prints the Case listings for SUSAR (suspected unexpected serious adverse reaction) occurrences that match report criteria. |
Note: Your Admin can configure custom SAR report templates for your organization.
Prerequisites
Your Admin must configure Workbench SAR reports.
SAR Criteria
Vault uses the SAR Principal view to assess each Case. To qualify for the SAR report, each returned Case must meet the following criteria:
- One (1) of the following are met:
- Contains at least one (1) Product defined by the product filter in the SAR Principal view.
- Contains at least one (1) Study defined by the study filter.
- The Case Category derived field is Interventional.
- Contains at least one (1) SAR. This is when at least one (1) event on the Case is serious and related to a Study Drug Role of IMP, Comparator, Placebo, or blank.
SUSAR Criteria
A SUSAR report provides a more specific subset of SAR data. To qualify for the SUSAR report, a Case must have at least one (1) Adverse Event that meets the following criteria:
- Is unlisted: Vault does the following to determine listedness:
- Identifies the relevant datasheet for the Case by ensuring the values for the following fields match between the datasheet and the Case Adverse Event:
- MedDRA Term
- Active Start Date
- Active End Date
- Identifies the Case as listed if at least one (1) MedDRA Term on the datasheet is expected. Vault identifies the Case as unlisted if all matching MedDRA Terms are unexpected.
- Identifies the relevant datasheet for the Case by ensuring the values for the following fields match between the datasheet and the Case Adverse Event:
- Is serious: Vault identifies an event as serious when the Seriousness field on the Case Adverse Event record is populated.
- Is related to the suspect Product: Vault identifies a Case Adverse Event as related to a Case Product when any Case Assessment Result for this pair has Causality Established set to Yes or blank.
Create a Workbench SAR Report
To create a Workbench SAR report:
- Navigate to Workbench > Ad-Hoc Reports.
- Select Create.
- Complete the following information:
- Name: Enter a name for this report.
- Workbench View: Select a SAR view from the drop-down or use the Advanced Search (
 ) icon to use filters and refine your search.
) icon to use filters and refine your search.
- Set up Workbench Report filters, layout, and advanced options (standard templates) as needed.
- Select Save.
- Run and download the SAR report.
Workbench SAR Table Generation Data Mapping
The following sections describe how Workbench generates SAR tabulations:
SAR Line Listing
The following images displays the Workbench SAR Line Listing:
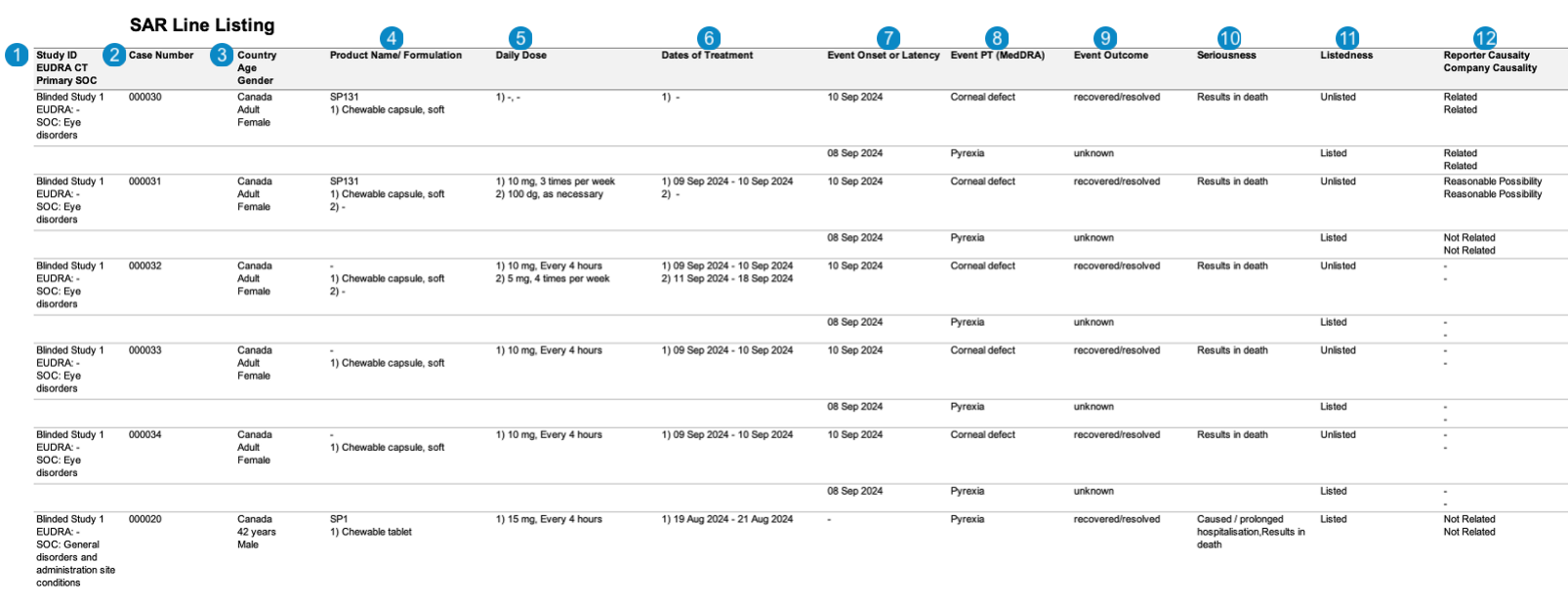
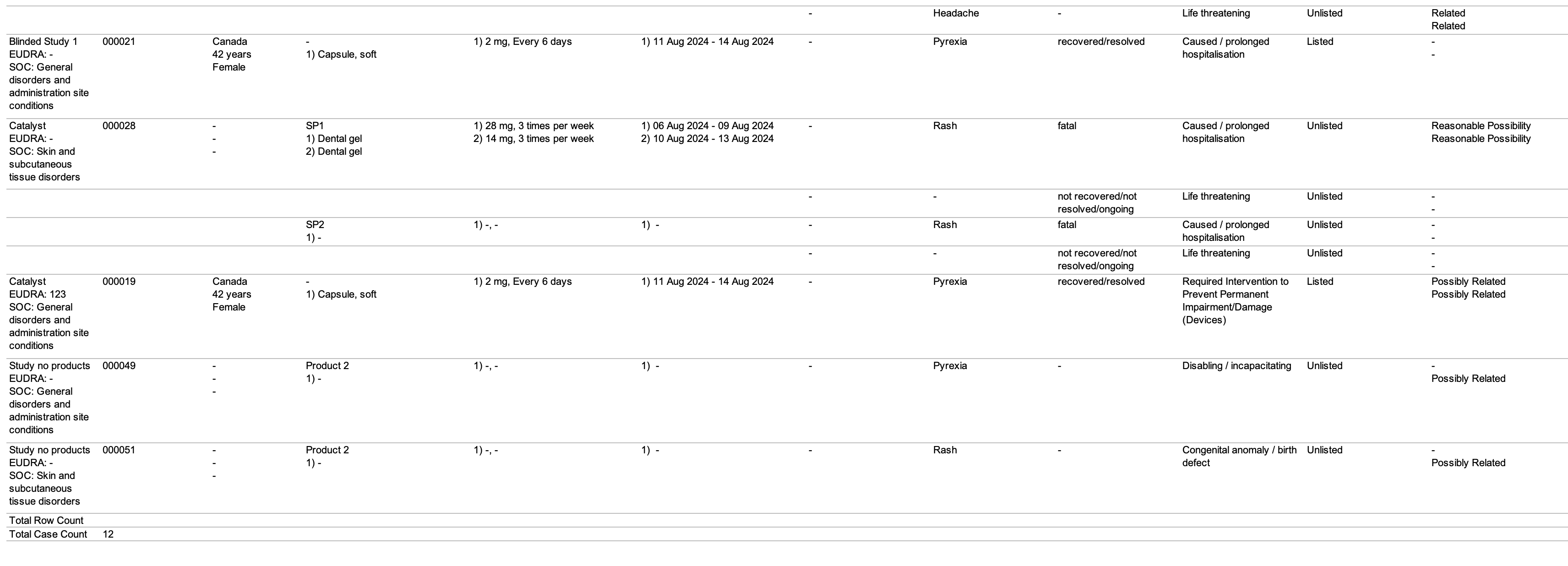
| Number | Name | Description |
|---|---|---|
| Study ID EUDRA CT Primary SOC |
Vault maps these values from the following fields:
|
|
| Case Number | Vault maps this value from the Case > Case Number field.case_version__v.case_number__v
|
|
| Country Age Gender |
Vault maps these values from the following fields:
|
|
| Product Name/Formulation | Vault maps these values from the following fields:
|
|
| Daily Dose | Vault maps these values from the following fields:
|
|
| Dates of Treatment | Vault maps these values from the Case Product Dosage > First Admin Date to Case Product Dosage > Last Admin Date in the format (DD-MMM-YYYY).case_product_dosage__v.firstadmin_normalized__v - case_product_dosage__v.lastadmin_normalized__v
|
|
| Event Onset or Latency | Vault maps this value from the Case Adverse Event > Onset field. If the Onset field is blank, Vault maps this value from the Case Assessment > First Dose Latency (number and unit) field. |
|
| Event PT (MedDRA) | The MedDRA PT of the Case Adverse Event.case_adverse_event__v.event_meddra__v.pt_term__v
|
|
| Event Outcome | Vault maps this value from the Case Adverse Event > Outcome field.case_adverse_event__v.event_outcomes__v.name__v
|
|
| Seriousness | The level of impact the adverse event had on the patient. Vault maps these values from the Case Adverse Event > Seriousness field.case_adverse_event__v.seriousness__v
|
|
| Listedness | Whether the Case is listed or unlisted. Vault sets the value to Vault sets the value to |
|
| Reporter Causality Company Causality |
The results of the Case Assessment for the Reporter and Company source type. Vault maps these values from the Case Assessment Result > Assessment Result field.case_assessment__v.case_assessment_result__v.assessment_result__v
|
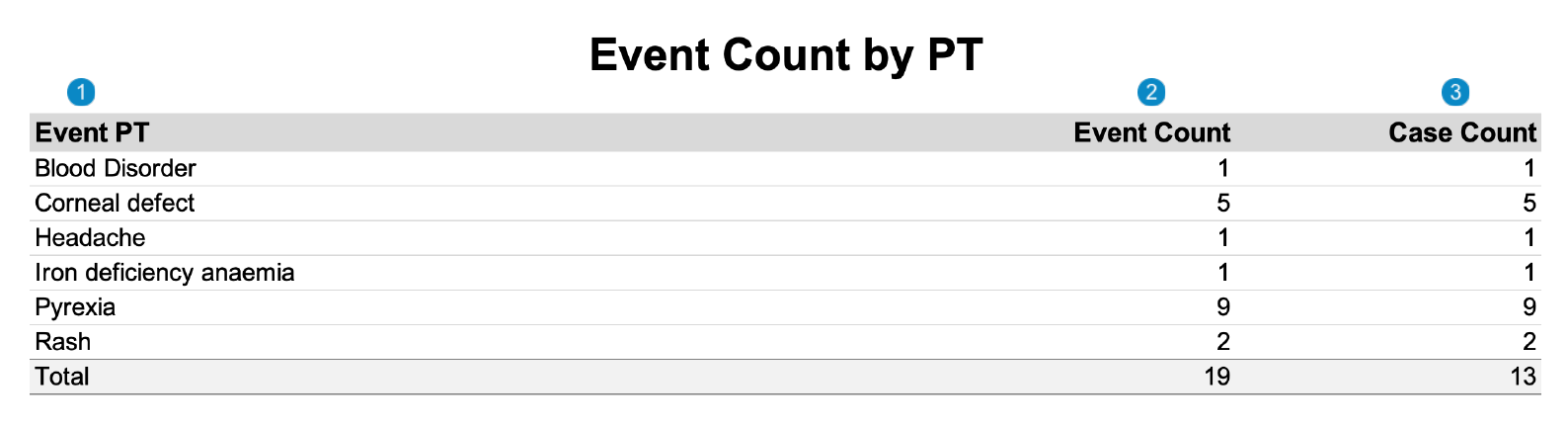
SAR Tabulation by PT
The following image displays the Workbench SAR Tabulation by PT:
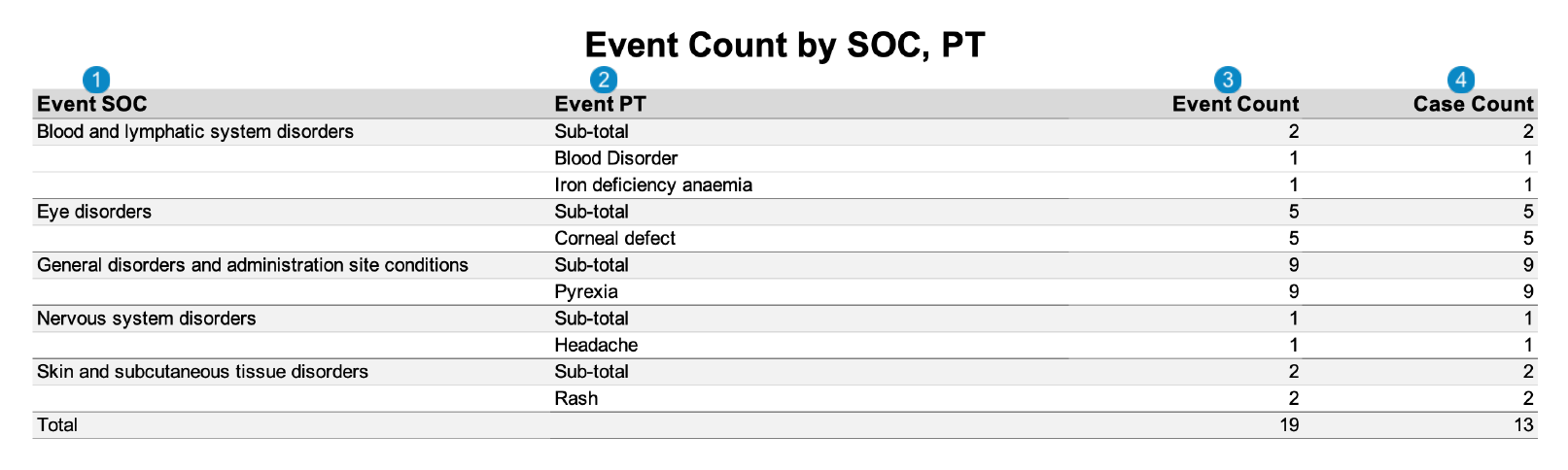
SAR Tabulation by SOC, PT
The following image displays the Workbench SAR Tabulation by SOC, PT:
SUSAR Line Listing
The following images displays the Workbench SUSAR Line Listing: