Workbench provides Development Safety Update Report (DSUR) table generation capabilities. Vault generates the following DSUR tabulations and line listings, with support for masked and unmasked versions of each:
- Cumulative Tabulation of Serious Adverse Events From Clinical Trials
- Interval Line Listings of Serious Adverse Reactions
- Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials
- List of Subjects Who Died During the Reporting Period
In addition to the standard DSUR tabulations and line listings, Vault can produce masked and unmasked versions of the following log-type reports:
Note: This article uses the generic term reports to refer to standard DSUR tabulations, line listings, and log-type reports.
Prerequisites
To generate Workbench DSUR aggregate reports:
- Your Admin must configure the following features:
- Your Admin must configure Study Products to generate table data from Study-type Cases
- Your Admin must grant you permissions to view and prepare aggregate reports
- Depending on your business process, your Admin may:
- Configure a Datasheet for each Study Product, Study, or Product with a list of expected adverse events in order to classify adverse events as Listed or Unlisted in Workbench DSUR reports
- Configure custom Workbench DSUR report templates for your organization
Overview
To generate a Workbench DSUR aggregate report:
- Create a Workbench Report Set to combine the desired DSUR Workbench Report Definitions into a single record. For example, you can create a Workbench Report Set that contains both masked and unmasked versions of each DSUR report.
- Specify the required DSUR filters on the Workbench Report Set.
- Generate then run the DSUR Workbench Reports from the Workbench Report Set.
To learn how Vault maps data to each Workbench DSUR report, see DSUR Table Generation Data Mapping.
Create a DSUR Workbench Report Set
To create a DSUR Workbench Report Set:
- Navigate to Workbench > Report Sets.
- Select Create.
- On the Create Workbench Report Set page, enter a name for the report.
- Select Save or Save + Create to save the Workbench Report Set and create another.
- In the Reports to Generate section, add a Workbench Report Definition to the Workbench Report Set for each DSUR report you want to include.
Note: When your Admin configures Workbench DSUR Aggregate Reports, they create a Workbench Report Definition for each masked and unmasked report. If your Workbench Report Set does not contain the expected DSUR reports, contact your Admin for assistance.
Result
Vault sets the lifecycle state of the Workbench Report Set to Draft.
Specify DSUR Workbench Report Set Filters
To specify Workbench Report Set filters for all Workbench Reports in the Workbench Report Set:
- Expand the Filters section and select Refresh.
- Select Edit.
- In the Filters section, populate the required filter fields.
- Select Save.
Filter Section Fields
The following fields may be available:
| Field | Description |
|---|---|
| Data Period Start |
Use the calendar to select the start date for the reporting period or enter the date manually. Vault uses this date, along with the Data Period End value, to determine the reporting period, which defines the Cases to include in generated interval (but not cumulative) reports. Vault includes Cases with a New Info Date value (or Receipt field value, if the New Info Date field is blank) that is on or within the specified reporting period. Note: If there are multiple versions of the Case within the reporting period, Vault lists only the most recent Case version. |
| Datasheet Active Date |
Use the calendar to select the date at which to consider Datasheets active or enter the date manually. Vault uses this date to determine adverse event term expectedness. If the Datasheet Active Date is outside of a term's active range, Vault considers that term unexpected and displays an asterisk (*) next to the term in the following reports, whether masked or unmasked: |
| Aggregate Reporting Group |
Select an Aggregate Reporting Group from the drop-down, or use the Advanced Search ( Vault considers the following Cases eligible for inclusion in the generated reports:
|
| Data Period End | Use the calendar to select the end date for the reporting period or enter the date manually. |
| Case Completed | Select whether the Case is open or completed from the drop-down. If you leave this field blank, the report filters for all Cases. |
Generate and Run a DSUR Workbench Report Set
After you have populated your Workbench Report Set with the relevant DSUR Workbench Report Definitions and you’ve specified the relevant DSUR filters, you can then generate and run all specified DSUR Workbench Reports from the Workbench Report Set.
DSUR Table Generation Data Mapping
When you generate DSUR Workbench Reports from a Workbench Report Set, Vault uses its Workbench Report Definitions to create Workbench Reports. After this point, whenever you run the Workbench Report Set, Vault creates CSV and Excel files for each Workbench Report and attaches these files to each Workbench Report. Vault populates these files with Cases that meet:
- The inclusion criteria for all Workbench DSUR reports
- The filtering criteria defined on the Workbench Report Set
- The inclusion criteria on the specific report
Inclusion Criteria for All Workbench DSUR Reports
Vault considers the following criteria when assessing Cases for inclusion in any Workbench DSUR report:
- Drug Roles: Vault considers Case Products with the following Drug Role values:
- Suspect
- Interacting
- Drug Not Administered
- Lifecycle States: Vault considers completed Cases1—that is, Cases with the following Lifecycle State values:
- Closed
- Approved
- Superseded
- Seriousness: Vault considers Cases with at least one (1) Case Adverse Event with a non-blank Seriousness value.
- Report Types and Study Types: Vault includes Cases that include the specified Report Type and Study Type values defined in either of the scenarios outlined in the following table.
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
- The Open Cases Report differs from this Lifecycle State filter logic. It includes Cases with any open Lifecycle State value.
Case Report Type and Study Type Inclusion Scenarios
Vault considers Cases that include the specified values in either of the following scenarios:
| Scenario | Report Type | Study Type |
|---|---|---|
| 1 |
|
|
|
|
||
| 2 |
|
|
|
|
||
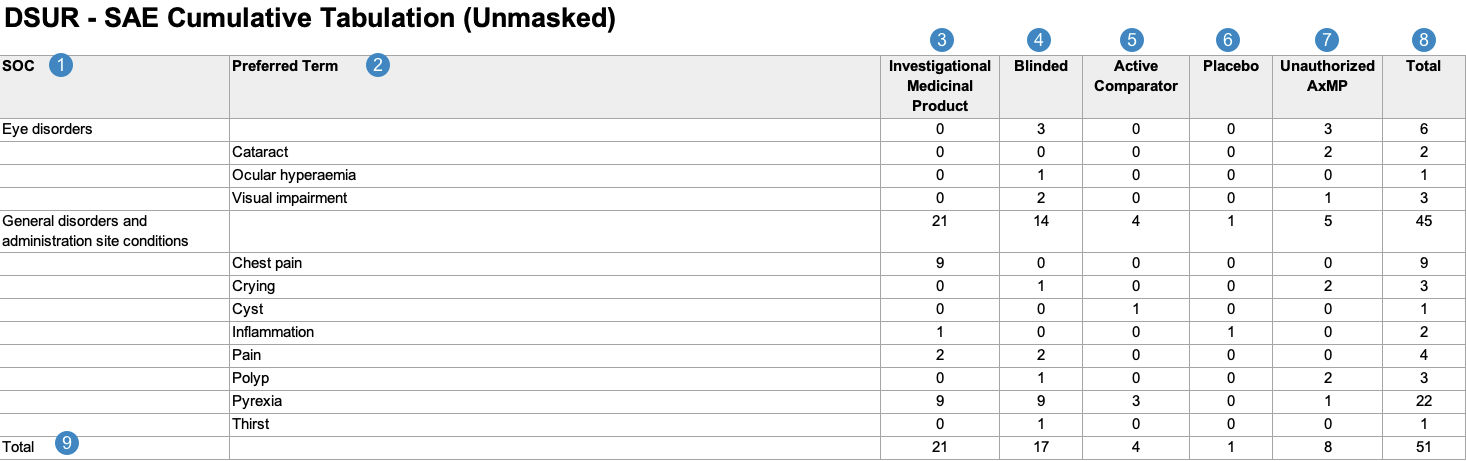
Cumulative Tabulation of Serious Adverse Events From Clinical Trials
When generating the Cumulative Tabulation of Serious Adverse Events from Clinical Trials, Vault uses the logic defined by your Admin in the corresponding Workbench View. By default, this generates report data in the following structure:
Note: To aid in understanding how Vault maps Cumulative Tabulation of Serious Adverse Events From Clinical Trials reporting data to DSUR files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | DSUR Report Field Name | Description |
|---|---|---|
| SOC |
Vault maps the MedDRA System Organ Class (SOC) for the adverse event from the SOC value on the MedDRA record linked to the Case Adverse Event (via the Event (LLT) field).
|
|
| Preferred Term |
Vault maps the MedDRA Preferred Term (MedDRA PT) for each Case Adverse Event, grouped by the MedDRA SOC.
Note: When a Case contains multiple Case Adverse Events coded under the same MedDRA PT, the report counts a single PT event instead of multiple events. |
|
| Investigational Medicinal Product |
Vault totals the number of Case Adverse Events with Study Case Products that have a Study Product Role value of Investigational.1
|
|
| Blinded |
Vault totals the number of Case Adverse Events with blinded Study Case Products.1
|
|
| Active Comparator |
Vault totals the number of Case Adverse Events with active comparators—that is, a Study Case Product with a Study Product Role value of Active Comparator.1
|
|
| Placebo |
Vault totals the number of Case Adverse Events with placebos—that is, a Study Case Product with a Study Product Role value of Placebo.1
|
|
| Unauthorized AxMP |
Vault totals the number of Case Adverse Events with unauthorized AxMPs1, as determined by the following:
Note: By default, Vault includes an Unauthorized AxMP column in this report per EMA regulations. If your Admin has not configured Auxiliary Medicinal Product Support, or if the Study does not include any unauthorized AxMPs, your Admin can remove this column from the corresponding Workbench Report Definition layout. |
|
| Total |
Vault maps the sum of the following types of Case Adverse Events for each SOC and PT:
|
|
| Total |
Vault maps the total number of Case Adverse Events for each of the following categories:
|
|
| 1. Although a Case Adverse Event may contain multiple Case Study Products that correspond to different columns in the Cumulative Tabulation of Serious Adverse Events From Clinical Trials, Vault only counts the Case Adverse Event in a single column, based on the product's causality. | ||
Case Inclusion Criteria
Vault determines which data to include in the Cumulative Tabulation of Serious Adverse Events From Clinical Trials based on the following constraining criteria:
- General DSUR report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- Seriousness
- Report Type and Study Type
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period End
- Aggregate Reporting Group
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
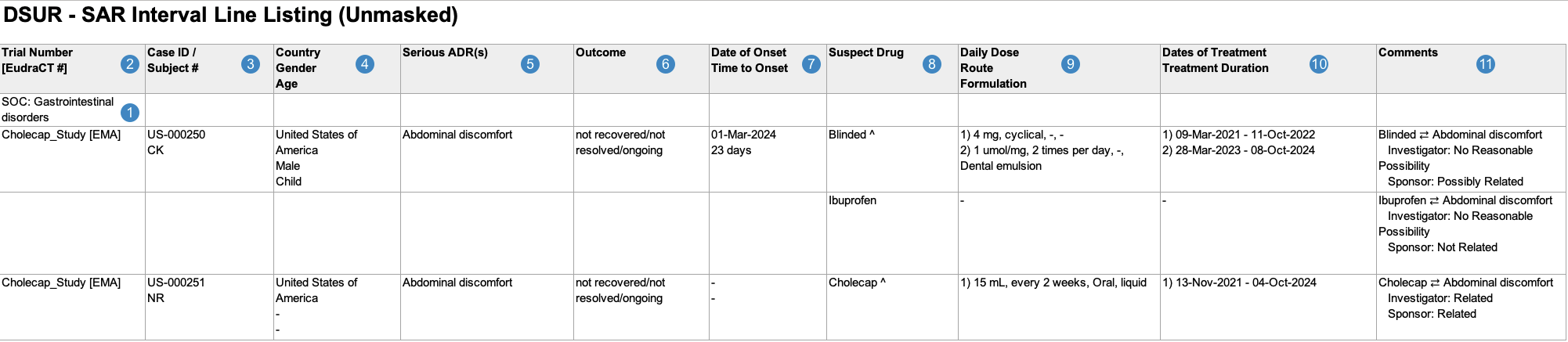
Interval Line Listings of Serious Adverse Reactions
When generating the Interval Line Listings of Serious Adverse Reactions, Vault uses the logic defined by your Admin in the corresponding Workbench View. By default, this generates report data in the following structure:
Note: To aid in understanding how Vault maps Interval Line Listings of Serious Adverse Reactions reporting data to DSUR files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | DSUR Report Field Name | Description |
|---|---|---|
| SOC |
Vault maps the MedDRA System Organ Class (SOC) for the adverse event from the SOC value on the MedDRA record linked to the Case Adverse Event (via the Event (LLT) field).
|
|
| Trial Number [EudraCT#] |
For Studies registered to a country in the European Union, Vault maps values from the following fields:
|
|
| Case ID/ Subject # |
Vault maps values from the following fields:
Note: Vault hides Subject ID values for CTIS Annual Safety Report submissions. |
|
| Country Gender Age |
Vault maps values from the following fields:
|
|
| Serious ADR(s) |
Vault maps the MedDRA Preferred Terms (MedDRA PTs) for each Case Adverse Event, ordered by Case Adverse Event rank.
If the Datasheet Active Date value on the Workbench Report Set is outside of a term's active range, Vault considers that term unexpected and displays an asterisk (*) next to the term, whether masked or unmasked. |
|
| Outcome |
Vault maps the Outcome value on the Case Adverse Event. If a Case contains multiple Case Adverse Events, Vault populates the most serious outcome, per E2B guidelines.
|
|
| Date of Onset Time to Onset |
Vault maps values from the following fields on the primary Case Adverse Event:
|
|
| Suspect Drug |
Vault maps the Product Names of all Case Products on the Case, ordered by rank. If a Case Product is serious, related, and investigational, Vault appends
|
|
| Daily Dose Route Formulation |
Vault populates the daily dose, route, and formulation data for all Case Products. If the Case Product has multiple Dosages, Vault numbers and lists all Dosages in the same cell, ordered by First Administration date. Vault maps all relevant Case Product Dosage values in a comma-separated list as follows:
|
|
| Dates of Treatment Treatment Duration |
Vault maps values for each Case Product > Case Product Dosage as follows:
|
|
| Comments |
For each combination of Suspect Drug(s) and Serious ADR(s) listed in the report, Vault populates a unique line in the cell with any Source Type, and Assessment Result values on the Case Assessment Result. Note: Vault maps Assessment Results in the same row as the suspect drug. The way Vault maps these values depends on which fields are populated:
Vault maps the value in the Reporting Summary field in only the first line in the cell. case_version__v.reporting_summary__v
|
Case Inclusion Criteria
Vault determines which data to include in the Interval Line Listings of Serious Adverse Reactions based on the following constraining criteria:
- General DSUR report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- Seriousness
- Report Type and Study Type
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period Start
- Data Period End
- Datasheet Active Date
- Aggregate Reporting Group
- Inclusion criteria specific to the Interval Line Listings of Serious Adverse Reactions—that is, investigational Study Product Role relatedness
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
Investigational Study Product Role Relatedness
Vault considers only Cases with at least one (1) serious adverse event that is conservatively related to an investigational Study Product. An investigational Study Product is a Study Product that has one (1) of the following Study Product Roles:
- Investigational
- Active Comparator
- Placebo
- Blinded
- Unauthorized AxMP
Vault assumes a product-event pair is conservatively related unless all Case Assessment Results are assessed as unrelated. In addition, in order for a product-event pair to be considered unrelated, the Case must contain:
- At least one (1) Case Assessment Result for the company (Sponsor or MAH)—that is, a Source Type value that maps to E2B Code
2or4 - At least one (1) other Case Assessment Result with a Source Type value that does not map to E2B Code
2or4
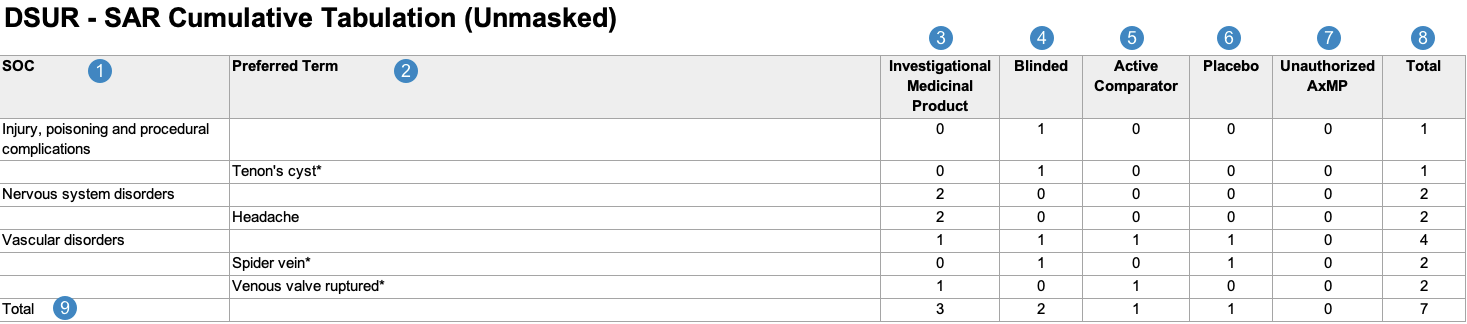
Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials
When generating the Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials, Vault uses the logic defined by your Admin in the corresponding Workbench View. By default, this generates report data in the following structure:
Note: To aid in understanding how Vault maps Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials reporting data to DSUR files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | DSUR Report Field Name | Description |
|---|---|---|
| SOC |
Vault maps the MedDRA System Organ Class (SOC) for the adverse event from the SOC value on the MedDRA record linked to the Case Adverse Event (via the Event (LLT) field).
|
|
| Preferred Term |
Vault maps the MedDRA Preferred Term (MedDRA PT) for the Case Adverse Event, provided at least one (1) Study Product is blank (unknown) or assessed as related to the Case Adverse Event. Vault groups all qualifying Case Adverse Events by their common MedDRA SOC. If the Datasheet Active Date value on the Workbench Report Set is outside of a term's active range, Vault considers that term unexpected and displays an asterisk (*) next to the term, whether masked or unmasked.
Note: When a Case contains multiple Case Adverse Events coded under the same MedDRA PT, the report counts a single PT event instead of multiple events. Vault excludes Case Adverse Events that contain multiple Case Assessment Results with a Causality Established value of No and:
|
|
| Investigational Medicinal Product |
Vault totals the number of Case Adverse Events with Study Case Products that have a Study Product Role value of Investigational.1
|
|
| Blinded |
Vault totals the number of Case Adverse Events with blinded Study Case Products.1
|
|
| Active Comparator |
Vault totals the number of Case Adverse Events with active comparators—that is, a Study Case Product with a Study Product Role value of Active Comparator.1
|
|
| Placebo |
Vault totals the number of Case Adverse Events with placebos—that is, a Study Case Product with a Study Product Role value of Placebo.1
|
|
| Unauthorized AxMP |
Vault totals the number of Case Adverse Events with unauthorized AxMPs1, as determined by the following:
Note: By default, Vault includes an Unauthorized AxMP column in this report per EMA regulations. If your Admin has not configured Auxiliary Medicinal Product Support, or if the Study does not include any unauthorized AxMPs, your Admin can remove this column from the corresponding Workbench Report Definition layout. |
|
| Total |
Vault maps the sum of the following types of Case Adverse Events for each SOC and PT:
|
|
| Total |
Vault maps the total number of Case Adverse Events for each of the following categories:
|
|
| 1. Although a Case Adverse Event may contain multiple Case Study Products that correspond to different columns in the Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials, Vault only counts the Case Adverse Event in a single column, based on the product's causality. | ||
Case Inclusion Criteria
Vault determines which data to include in the Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials based on the following constraining criteria:
- General DSUR report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- Seriousness
- Report Type and Study Type
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period Start
- Data Period End
- Datasheet Active Date
- Aggregate Reporting Group
- Inclusion criteria specific to the Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials—that is, investigational Study Product Role relatedness
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
Investigational Study Product Role Relatedness
Vault considers only Cases with at least one (1) serious adverse event that is conservatively related to an investigational Study Product. An investigational Study Product is a Study Product that has one (1) of the following Study Product Roles:
- Investigational
- Active Comparator
- Placebo
- Blinded
- Unauthorized AxMP
Vault assumes a product-event pair is conservatively related unless all Case Assessment Results are assessed as unrelated. In addition, in order for a product-event pair to be considered unrelated, the Case must contain:
- At least one (1) Case Assessment Result for the company (Sponsor or MAH)—that is, a Source Type value that maps to E2B Code
2or4 - At least one (1) other Case Assessment Result with a Source Type value that does not map to E2B Code
2or4
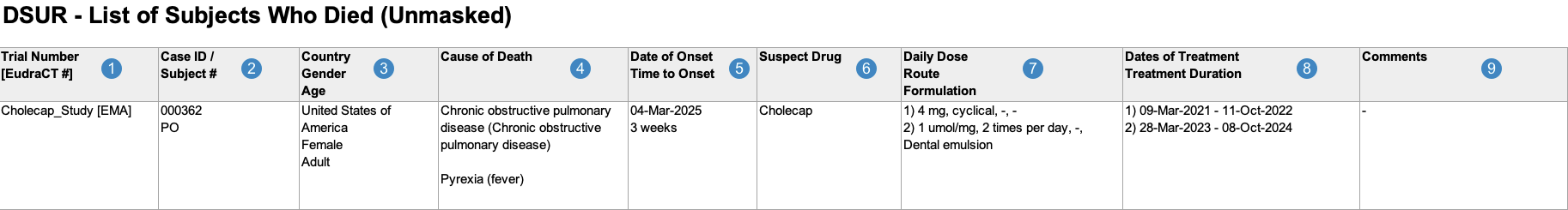
List of Subjects Who Died During the Reporting Period
When generating the List of Subjects Who Died During the Reporting Period, Vault uses the logic defined by your Admin in the corresponding Workbench View. By default, this generates report data in the following structure:
Note: To aid in understanding how Vault maps List of Subjects Who Died During the Reporting Period reporting data to DSUR files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | DSUR Report Field Name | Description |
|---|---|---|
| Trial Number [EudraCT#] |
For Studies registered to a country in the European Union, Vault maps values from the following fields:
|
|
| Case ID/ Subject # |
Vault maps values from the following fields:
Note: Vault hides Subject ID values for CTIS Annual Safety Report submissions. |
|
| Country Gender Age |
Vault maps values from the following fields:
|
|
| Cause of Death |
Vault maps the MedDRA Preferred Terms (Cause of Death PTs) associated with each Cause of Death record on the Case, followed by the verbatim Cause of Death (Reported) value enclosed in brackets.
If a Case contains multiple Case Causes of Death, Vault maps the values in the same cell, separated by a line break. |
|
| Date of Onset Time to Onset |
Vault maps values from the following fields on the Case Adverse Event:
|
|
| Suspect Drug |
Vault maps the Product Names of all Case Products on the Case, ordered by rank.
|
|
| Daily Dose Route Formulation |
Vault populates the daily dose, route, and formulation data for all Case Products. If the Case Product has multiple Dosages, Vault numbers and lists all Dosages in the same cell, ordered by First Administration date. Vault maps all relevant Case Product Dosage values in a comma-separated list as follows:
|
|
| Dates of Treatment Treatment Duration |
Vault maps values for each Case Product > Case Product Dosage as follows:
|
|
| Comments |
Vault maps the value in the Reporting Summary field on the Case.
|
Case Inclusion Criteria
Vault determines which data to include in the List of Subjects Who Died During the Reporting Period based on the following constraining criteria:
- General DSUR report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- Seriousness
- Report Type and Study Type
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period Start
- Data Period End
- Aggregate Reporting Group
- Inclusion criteria specific to the List of Subjects Who Died During the Reporting Period—that is, Cases that indicate a death has occurred
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
Case Indicates a Death Occurred
For Vault to consider a Case for the List of Subjects Who Died During the Reporting Period, it must meet one of the following conditions:
- The Case contains a Date of Death value
case_version__v.dod_normalized__v ≠ Blank - The Case contains a Seriousness value of Results in Death
case_version__v.seriousness__v = results_in_death__v - The Case contains an Autopsy value
case_version__v.autopsy_value__v = ≠ Blank - The Case contains a Case Cause of Death record
case_version__v.case_cause_of_death__v ≠ 0 - The Case contains any Case Adverse Event with an Outcome value of Fatal
case_version__v.case_adverse_event__v.outcome__v = fatal - The Case contains any Case Adverse Event linked to a MedDRA record with an HLT Code value of 10011907 (Death and Sudden Death)
case_adverse_event__v.event_meddra__v.meddra__v.hlt_code__v = 10011907
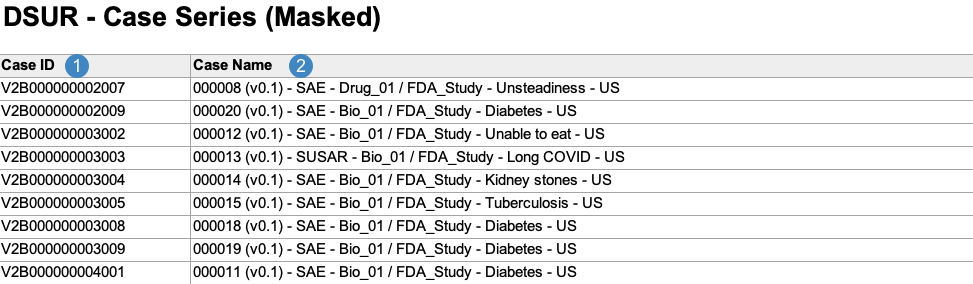
Case Series Report
The Case Series Report acts as a log file for all of the Cases included across all DSUR line listings and tabulations. Vault generates the Case Series Report in the following structure:
Note: To aid in understanding how Vault maps Case Series Report data to DSUR files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | DSUR Report Field Name | Description |
|---|---|---|
| Case ID |
Vault maps the Case ID on the Case.
|
|
| Case Name |
Vault maps the Name on the Case.
|
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
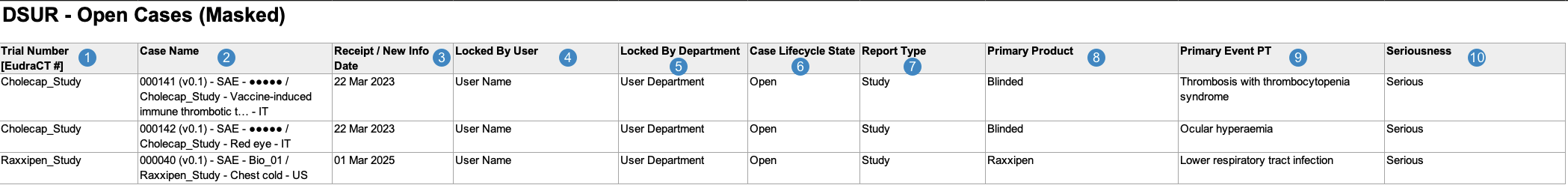
Open Cases Report
The Open Cases Report is an operational report intended to help you identify open Cases that may need to be closed before DSUR submission. It contains a log of all Cases that would be included in at least one DSUR tabulation were they in the Closed, Approved, or Superseded lifecycle state.
Vault generates the Open Cases Report in the following structure:
Note: To aid in understanding how Vault maps Open Cases Report data to DSUR files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | DSUR Report Field Name | Description |
|---|---|---|
| Trial Number [EudraCT#] |
For Studies registered to a country in the European Union, Vault maps values from the following fields:
|
|
| Case Name |
Vault maps the Name on the Case.
|
|
| Receipt / New Info Date |
Vault maps the New Info Date value from the Case (or Receipt field value, if the New Info Date field is blank).
|
|
| Locked By User |
Vault maps the Name value of the Locked By user on the Case.
|
|
| Locked By Department |
Vault maps the Department value of the Locked By user on the Case.
|
|
| Case Lifecycle State |
Vault populates
|
|
| Report Type |
Vault maps the Report Type value on the Case.
|
|
| Primary Product |
Vault maps the name of the primary Case Product on the Case.
|
|
| Primary Event PT |
Vault maps the MedDRA Preferred Term (MedDRA PT) for the primary Case Adverse Event.
|
|
| Seriousness |
Vault maps whether the Case is serious.
|
Case Inclusion Criteria
Vault determines which data to include in the Open Cases Report based on the following constraining criteria:
- General DSUR report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- Report Type and Study Type
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period End
- Aggregate Reporting Group
Note: Unlike the other DSUR reports, the Open Cases Report includes Cases with any open Lifecycle State value. However, it still excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes