Workbench provides Periodic Benefit-Risk Evaluation Report (PBRER) table generation capabilities. Vault generates the following standard PBRER tabulations and line listings, with support for masked and unmasked versions of each:
- Summary Tabulation of ADRs from Postmarketing Sources
- Cumulative Tabulation of Serious Adverse Events From Clinical Trials
- Interval Line Listings of Serious Adverse Reactions
In addition to the standard PBRER tabulations and line listings, Vault can produce masked and unmasked versions of the following log-type reports:
Note: This article uses the generic term reports to refer to standard DSUR tabulations, line listings, and log-type reports.
Prerequisites
To generate Workbench PBRER aggregate reports:
- Your Admin must configure the following features:
- Your Admin must configure Study Products to generate table data from Study-type Cases
- Your Admin must grant you permissions to view and prepare aggregate reports
- Depending on your business process, your Admin may:
- Configure a Datasheet for each Study Product, Study, or Product with a list of expected adverse events in order to classify adverse events as Listed or Unlisted in Workbench PBRER reports
- Configure custom Workbench PBRER report templates for your organization
Overview
To generate a Workbench PBRER aggregate report:
- Create a Workbench Report Set to combine the desired PBRER Workbench Report Definitions into a single record. For example, you can create a Workbench Report Set that contains both masked and unmasked versions of each PBRER report.
- Specify the required PBRER filters on the Workbench Report Set.
- Generate then run the PBRER Workbench Reports from the Workbench Report Set.
To learn how Vault maps data to each Workbench PBRER report, see PBRER Table Generation Data Mapping.
Create a PBRER Workbench Report Set
To create a PBRER Workbench Report Set:
- Navigate to Workbench > Report Sets.
- Select Create.
- On the Create Workbench Report Set page, enter a name for the report.
- Select Save or Save + Create to save the Workbench Report Set and create another.
- In the Reports to Generate section, add a Workbench Report Definition to the Workbench Report Set for each PBRER report you want to include.
Note: When your Admin configures Workbench PBRER Aggregate Reports, they create a Workbench Report Definition for each masked and unmasked report. If your Workbench Report Set does not contain the expected PBRER reports, contact your Admin for assistance.
Result
Vault sets the lifecycle state of the Workbench Report Set to Draft.
Specify PBRER Workbench Report Set Filters
To specify Workbench Report Set filters for all Workbench Reports in the Workbench Report Set:
- Expand the Filters section and select Refresh.
- Select Edit.
- In the Filters section, populate the required filter fields.
- Select Save.
Filter Section Fields
The following fields may be available:
| Field | Description |
|---|---|
| Data Period Start |
Use the calendar to select the start date for the reporting period or enter the date manually. Vault uses this date, along with the Data Period End value, to determine the reporting period, which defines the Cases to include in generated interval (but not cumulative) reports. Vault includes Cases with a New Info Date value (or Receipt field value, if the New Info Date field is blank) that is on or within the specified reporting period. Note: If there are multiple versions of the Case within the reporting period, Vault lists only the most recent Case version. |
| Datasheet Active Date |
Use the calendar to select the date at which to consider Datasheets active or enter the date manually. Vault uses this date to determine adverse event term expectedness. If the Datasheet Active Date is outside of a term's active range, Vault considers that term unexpected and displays an asterisk (*) next to the term in the following report, whether masked or unmasked: |
| Aggregate Reporting Group |
Select an Aggregate Reporting Group from the drop-down, or use the Advanced Search ( Vault considers the following Cases eligible for inclusion in the generated reports:
|
| Data Period End | Use the calendar to select the end date for the reporting period or enter the date manually. |
| Case Completed | Select whether the Case is open or completed from the drop-down. If you leave this field blank, the report filters for all Cases. |
Generate and Run a PBRER Workbench Report Set
After you have populated your Workbench Report Set with the relevant PBRER Workbench Report Definitions and you’ve specified the relevant PBRER filters, you can then generate and run all specified PBRER Workbench Reports from the Workbench Report Set.
PBRER Table Generation Data Mapping
When you generate PBRER Workbench Reports from a Workbench Report Set, Vault uses its Workbench Report Definitions to create Workbench Reports. After this point, whenever you run the Workbench Report Set, Vault creates CSV and Excel files for each Workbench Report and attaches these files to each Workbench Report. Vault populates these files with Cases that meet:
- The inclusion criteria for all Workbench PBRER reports
- The filtering criteria defined on the Workbench Report Set
- The inclusion criteria on the specific report
Inclusion Criteria for All Workbench PBRER Reports
Vault considers the following criteria when assessing Cases for inclusion in any Workbench PBRER report:
- Drug Roles: Vault considers Case Products with the following Drug Role values:
- Suspect
- Interacting
- Drug Not Administered
- Lifecycle States: Vault considers completed Cases1—that is, Cases with the following Lifecycle State values:
- Closed
- Approved
- Superseded
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
- The Open Cases Report differs from this Lifecycle State filter logic. It includes Cases with any open Lifecycle State value.
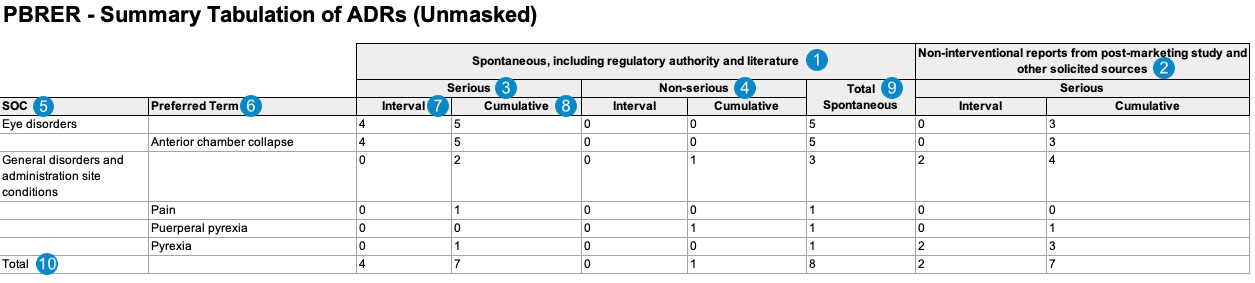
Summary Tabulation of Adverse Drug Reactions from Postmarketing Sources
When generating the Summary Tabulation of Adverse Drug Reactions from Postmarketing Sources, Vault uses the logic defined by your Admin in the corresponding Workbench View. By default, this generates report data in the following structure:
Note: To aid in understanding how Vault maps Summary Tabulation of Adverse Drug Reactions from Postmarketing Sources reporting data to PBRER files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | PBRER Report Field Name | Description | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spontaneous, including regulatory authority and literature | Cases are listed in this category when the Case Report Type is set to one of the following:
case_version__v.report_type__v = 1, 3, 4
|
|||||||||||||
| Non-interventional | Cases are listed in this category when they match one of the following scenarios:
COUNT IF
|
|||||||||||||
| Serious | Number of adverse events with a value entered in the Case Adverse Event Seriousness field.
case_adverse_event__v.seriousness__v ≠ EMPTY |
|||||||||||||
| Non-Serious | Number of adverse events with an empty Case Adverse Event Seriousness field.
case_adverse_event__v.serious__v = EMPTY |
|||||||||||||
| SOC |
Vault maps the MedDRA System Organ Class (SOC) for the adverse event from the SOC value on the MedDRA record linked to the Case Adverse Event (via the Event (LLT) field).
|
|||||||||||||
| Preferred Term |
Vault maps the MedDRA Preferred Term (MedDRA PT) for each Case Adverse Event, grouped by the MedDRA SOC.
Note: When a Case contains multiple Case Adverse Events coded under the same MedDRA PT, the report counts a single PT event instead of multiple events. |
|||||||||||||
| Interval |
The number of adverse events with a Case date within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
If the Case New Info Date is blank, Vault uses the Receipt Date:
Otherwise, Vault uses the New Info Date:
|
|||||||||||||
| Cumulative |
The number of adverse events with a Case date within the aggregate report cumulative reporting period (up to the Data Period End). How Aggregate Reports Filter by Data Period provides more information.
If the Case New Info Date is blank, Vault uses the Receipt Date:
Otherwise, Vault uses the New Info Date:
|
|||||||||||||
| Total Spontaneous | The total number of all adverse events within the "Spontaneous, including regulatory authority and literature" category, including both serious and non-serious, within the cumulative reporting period. | |||||||||||||
| Total | The total number of adverse events for each category within the reporting period. |
Case Inclusion Criteria
Vault determines which data to include in the Summary Tabulation of Adverse Drug Reactions from Postmarketing Sources based on the following constraining criteria:
- General PBRER report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period Start
- Data Period End
- Aggregate Reporting Group
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
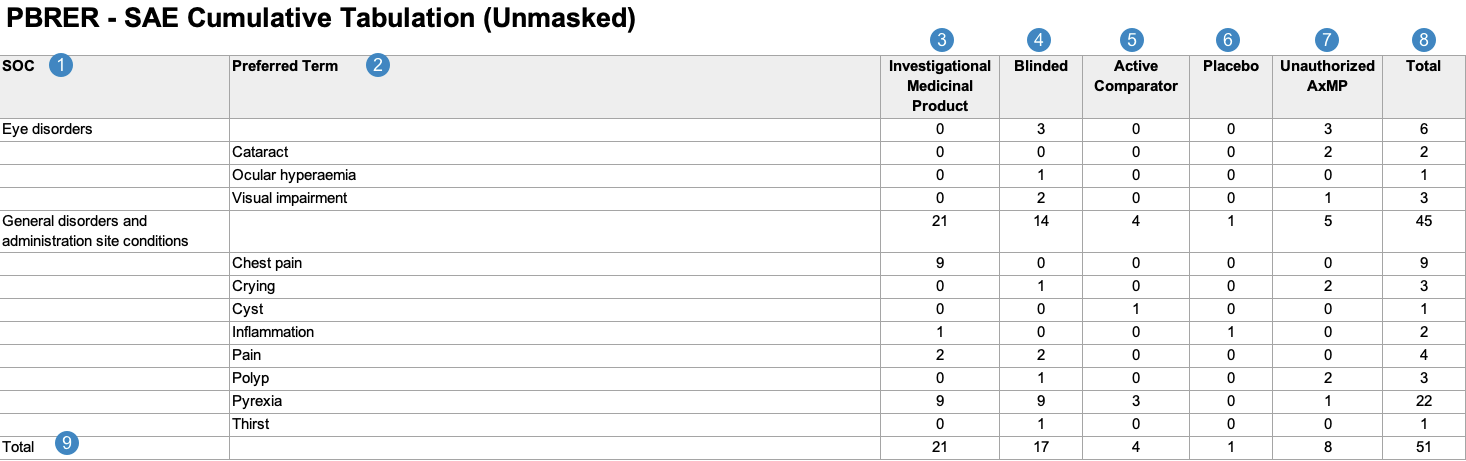
Cumulative Tabulation of Serious Adverse Events From Clinical Trials
When generating the Cumulative Tabulation of Serious Adverse Events from Clinical Trials, Vault uses the logic defined by your Admin in the corresponding Workbench View. By default, this generates report data in the following structure:
Note: To aid in understanding how Vault maps Cumulative Tabulation of Serious Adverse Events From Clinical Trials reporting data to PBRER files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | PBRER Report Field Name | Description |
|---|---|---|
| SOC |
Vault maps the MedDRA System Organ Class (SOC) for the adverse event from the SOC value on the MedDRA record linked to the Case Adverse Event (via the Event (LLT) field).
|
|
| Preferred Term |
Vault maps the MedDRA Preferred Term (MedDRA PT) for each Case Adverse Event, grouped by the MedDRA SOC.
Note: When a Case contains multiple Case Adverse Events coded under the same MedDRA PT, the report counts a single PT event instead of multiple events. |
|
| Investigational Medicinal Product |
Vault totals the number of Case Adverse Events with Study Case Products that have a Study Product Role value of Investigational.1
|
|
| Blinded |
Vault totals the number of Case Adverse Events with blinded Study Case Products.1
|
|
| Active Comparator |
Vault totals the number of Case Adverse Events with active comparators—that is, a Study Case Product with a Study Product Role value of Active Comparator.1
|
|
| Placebo |
Vault totals the number of Case Adverse Events with placebos—that is, a Study Case Product with a Study Product Role value of Placebo.1
|
|
| Unauthorized AxMP |
Vault totals the number of Case Adverse Events with unauthorized AxMPs1, as determined by the following:
Note: By default, Vault includes an Unauthorized AxMP column in this report per EMA regulations. If your Admin has not configured Auxiliary Medicinal Product Support, or if the Study does not include any unauthorized AxMPs, your Admin can remove this column from the corresponding Workbench Report Definition layout. |
|
| Total |
Vault maps the sum of the following types of Case Adverse Events for each SOC and PT:
|
|
| Total |
Vault maps the total number of Case Adverse Events for each of the following categories:
|
|
| 1. Although a Case Adverse Event may contain multiple Case Study Products that correspond to different columns in the Cumulative Tabulation of Serious Adverse Events From Clinical Trials, Vault only counts the Case Adverse Event in a single column, based on the product's causality. | ||
Case Inclusion Criteria
Vault determines which data to include in the Cumulative Tabulation of Serious Adverse Events From Clinical Trials based on the following constraining criteria:
- General PBRER report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period End
- Aggregate Reporting Group
- Inclusion criteria specific to the Cumulative Tabulation of Serious Adverse Events from Clinical Trials—that is:
- Seriousness: Vault considers Cases with at least one (1) Case Adverse Event with a non-blank Seriousness value
- Case Category: See the Case Report Type and Study Type Inclusion Scenarios table below
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
Case Report Type and Study Type Inclusion Scenarios
Vault considers Cases that include the specified values in either of the following scenarios:
| Scenario | Report Type | Study Type |
|---|---|---|
| 1 |
|
|
|
|
||
| 2 |
|
|
|
|
||
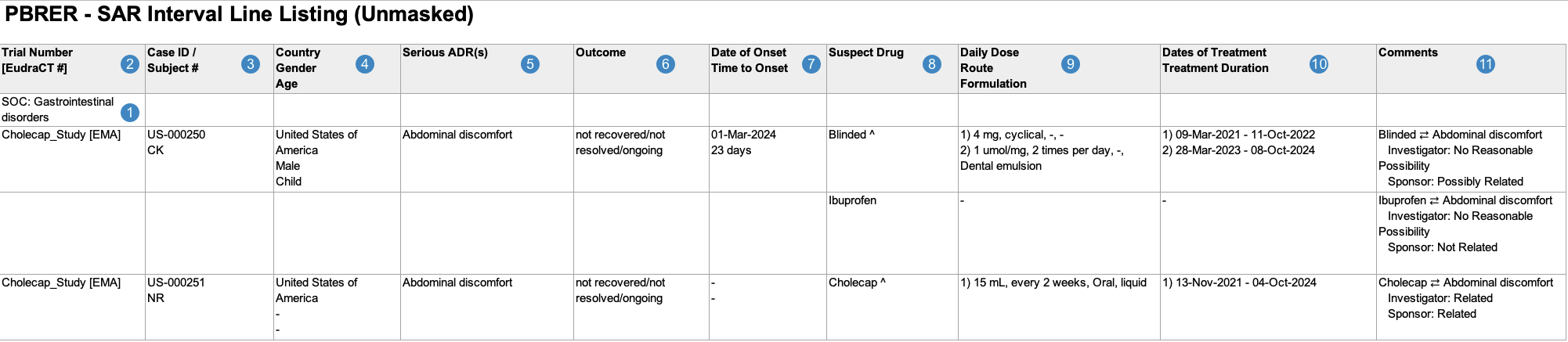
Interval Line Listings of Serious Adverse Reactions
When generating the Interval Line Listings of Serious Adverse Reactions, Vault uses the logic defined by your Admin in the corresponding Workbench View. By default, this generates report data in the following structure:
Note: To aid in understanding how Vault maps Interval Line Listings of Serious Adverse Reactions reporting data to PBRER files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | PBRER Report Field Name | Description |
|---|---|---|
| SOC |
Vault maps the MedDRA System Organ Class (SOC) for the adverse event from the SOC value on the MedDRA record linked to the Case Adverse Event (via the Event (LLT) field).
|
|
| Trial Number [EudraCT#] |
For Studies registered to a country in the European Union, Vault maps values from the following fields:
|
|
| Case ID/ Subject # |
Vault maps values from the following fields:
Note: Vault hides Subject ID values for CTIS Annual Safety Report submissions. |
|
| Country Gender Age |
Vault maps values from the following fields:
|
|
| Serious ADR(s) |
Vault maps the MedDRA Preferred Terms (MedDRA PTs) for each Case Adverse Event, ordered by Case Adverse Event rank.
If the Datasheet Active Date value on the Workbench Report Set is outside of a term's active range, Vault considers that term unexpected and displays an asterisk (*) next to the term, whether masked or unmasked. |
|
| Outcome |
Vault maps the Outcome value on the Case Adverse Event. If a Case contains multiple Case Adverse Events, Vault populates the most serious outcome, per E2B guidelines.
|
|
| Date of Onset Time to Onset |
Vault maps values from the following fields on the primary Case Adverse Event:
|
|
| Suspect Drug |
Vault maps the Product Names of all Case Products on the Case, ordered by rank. If a Case Product is serious, related, and investigational, Vault appends
|
|
| Daily Dose Route Formulation |
Vault populates the daily dose, route, and formulation data for all Case Products. If the Case Product has multiple Dosages, Vault numbers and lists all Dosages in the same cell, ordered by First Administration date. Vault maps all relevant Case Product Dosage values in a comma-separated list as follows:
|
|
| Dates of Treatment Treatment Duration |
Vault maps values for each Case Product > Case Product Dosage as follows:
|
|
| Comments |
For each combination of Suspect Drug(s) and Serious ADR(s) listed in the report, Vault populates a unique line in the cell with any Source Type, and Assessment Result values on the Case Assessment Result. Note: Vault maps Assessment Results in the same row as the suspect drug. The way Vault maps these values depends on which fields are populated:
Vault maps the value in the Reporting Summary field in only the first line in the cell. case_version__v.reporting_summary__v
|
Case Inclusion Criteria
Vault determines which data to include in the Interval Line Listings of Serious Adverse Reactions based on the following constraining criteria:
- General PBRER report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period Start
- Data Period End
- Datasheet Active Date
- Aggregate Reporting Group
- Inclusion criteria specific to the Interval Line Listings of Serious Adverse Reactions:
- Serious & Related Adverse Event: See investigational Study Product Role relatedness
- Case Category: See the Case Report Type and Study Type Inclusion Scenarios table below
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
Investigational Study Product Role Relatedness
Vault considers only Cases with at least one (1) serious adverse event that is conservatively related to an investigational Study Product. An investigational Study Product is a Study Product that has one (1) of the following Study Product Roles:
- Investigational
- Active Comparator
- Placebo
- Blinded
- Unauthorized AxMP
Vault assumes a product-event pair is conservatively related unless all Case Assessment Results are assessed as unrelated. In addition, in order for a product-event pair to be considered unrelated, the Case must contain:
- At least one (1) Case Assessment Result for the company (Sponsor or MAH)—that is, a Source Type value that maps to E2B Code
2or4 - At least one (1) other Case Assessment Result with a Source Type value that does not map to E2B Code
2or4
Case Report Type and Study Type Inclusion Scenarios
Vault considers Cases that include the specified values in either of the following scenarios:
| Scenario | Report Type | Study Type |
|---|---|---|
| 1 |
|
|
|
|
||
| 2 |
|
|
|
|
||
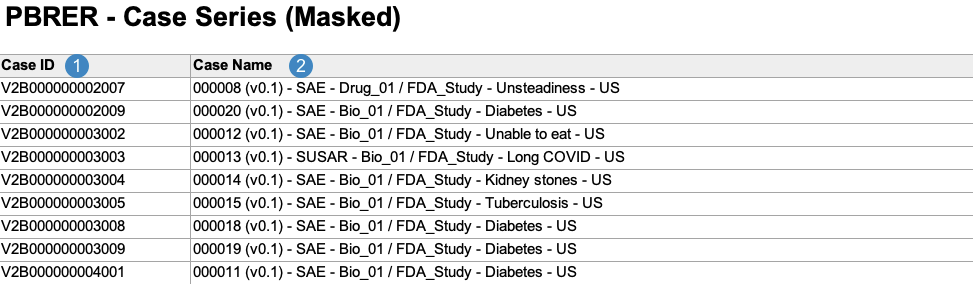
Case Series Report
The Case Series Report acts as a log file for all of the Cases included across all PBRER line listings and tabulations. Vault generates the Case Series Report in the following structure:
Note: To aid in understanding how Vault maps Case Series Report data to PBRER files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | PBRER Report Field Name | Description |
|---|---|---|
| Case ID |
Vault maps the Case ID on the Case.
|
|
| Case Name |
Vault maps the Name on the Case.
|
Note: Vault excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes
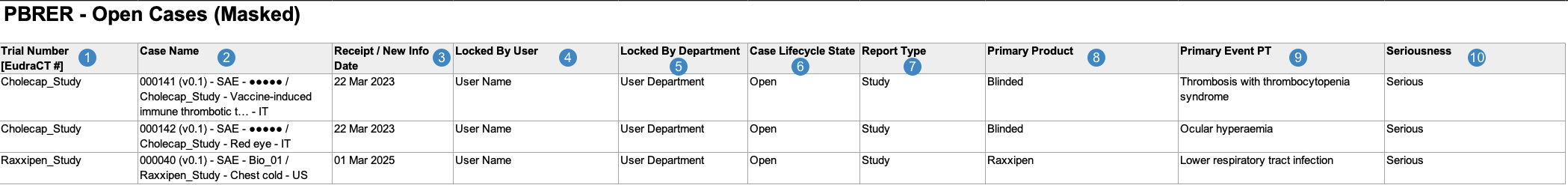
Open Cases Report
The Open Cases Report is an operational report intended to help you identify open Cases that may need to be closed before PBRER submission. It contains a log of all Cases that would be included in at least one PBRER tabulation were they in the Closed, Approved, or Superseded lifecycle state.
Vault generates the Open Cases Report in the following structure:
Note: To aid in understanding how Vault maps Open Cases Report data to PBRER files, the following table includes both descriptions and Vault object and field names.
| Reference Image Number | PBRER Report Field Name | Description |
|---|---|---|
| Trial Number [EudraCT#] |
For Studies registered to a country in the European Union, Vault maps values from the following fields:
|
|
| Case Name |
Vault maps the Name on the Case.
|
|
| Receipt / New Info Date |
Vault maps the New Info Date value from the Case (or Receipt field value, if the New Info Date field is blank).
|
|
| Locked By User |
Vault maps the Name value of the Locked By user on the Case.
|
|
| Locked By Department |
Vault maps the Department value of the Locked By user on the Case.
|
|
| Case Lifecycle State |
Vault populates
|
|
| Report Type |
Vault maps the Report Type value on the Case.
|
|
| Primary Product |
Vault maps the name of the primary Case Product on the Case.
|
|
| Primary Event PT |
Vault maps the MedDRA Preferred Term (MedDRA PT) for the primary Case Adverse Event.
|
|
| Seriousness |
Vault maps whether the Case is serious.
|
Case Inclusion Criteria
Vault determines which data to include in the Open Cases Report based on the following constraining criteria:
- General PBRER report Case filtering criteria—that is, specific values for:
- Drug Roles
- Lifecycle States
- Report Type and Study Type
- The filters defined on the Workbench Report Set—that is, specific values for:
- Data Period End
- Aggregate Reporting Group
Note: Unlike the other PBRER reports, the Open Cases Report includes Cases with any open Lifecycle State value. However, it still excludes Cases that contain the following field values:
- A Lifecycle State value of Nullified or Voided
- A Suppress Submission value of Yes