Sections in This Article
What’s Changing
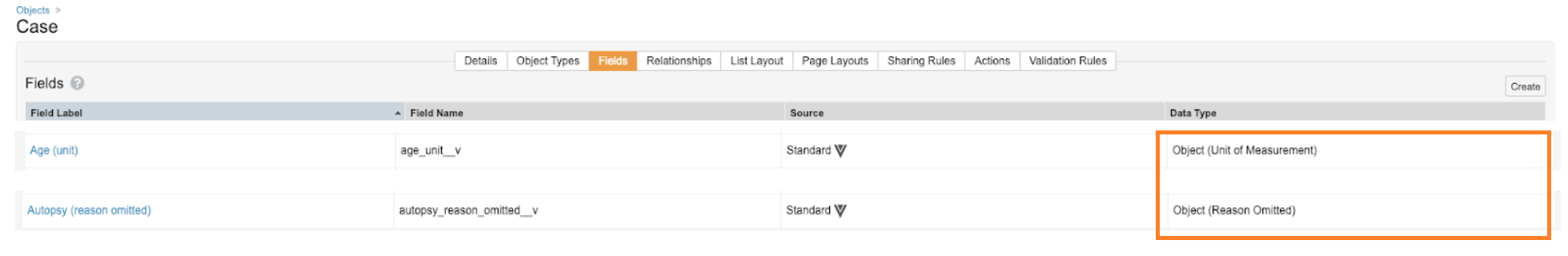
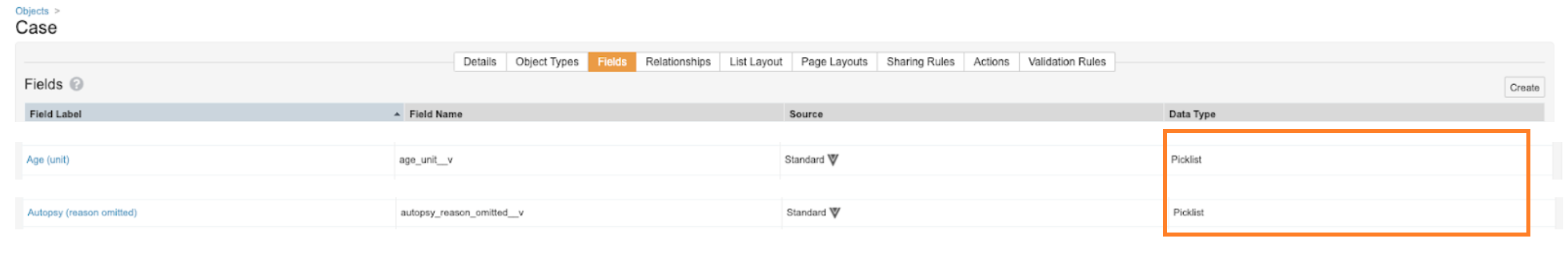
All Units of Measurement and Reason Omitted fields on all objects are being changed from object-type to picklist-type, specifically:
- Object (Unit of Measurement)
- Object (Reason Omitted)
Before
After
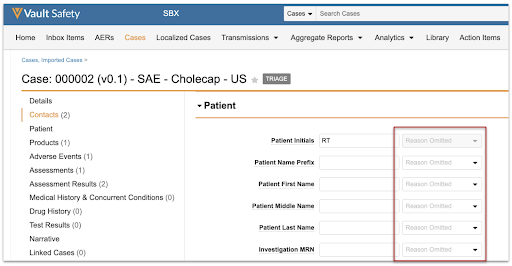
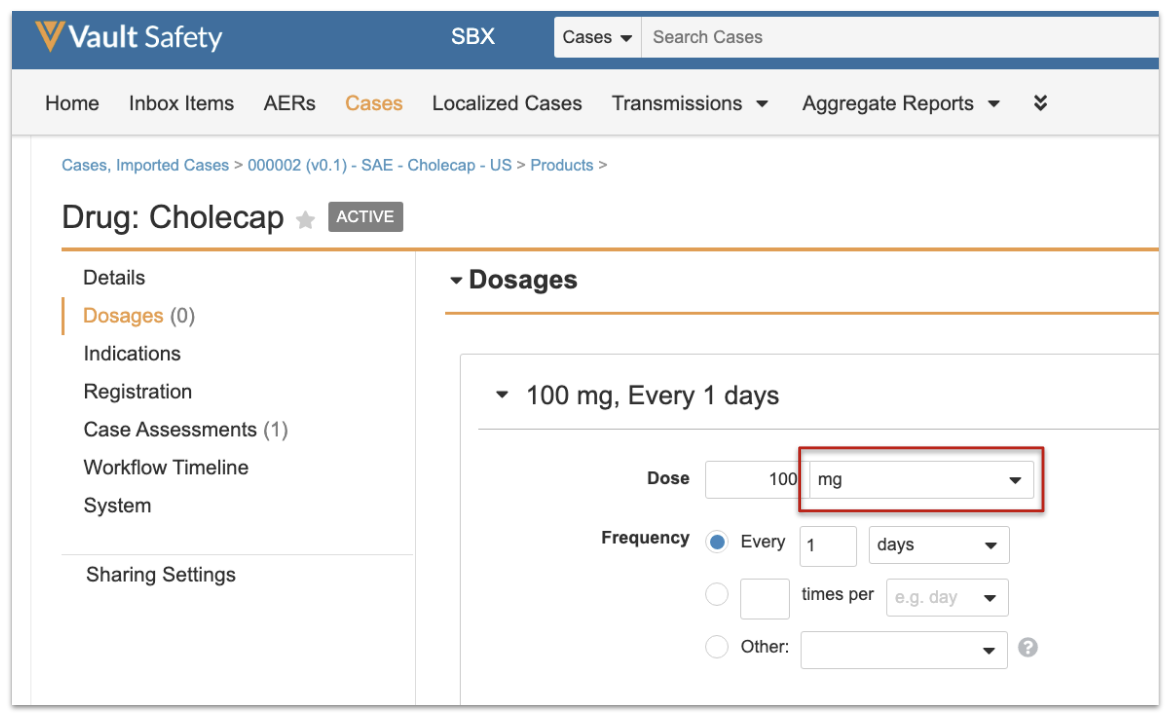
This update will be seamless to all users, as shown in the following images:
Why is this Changing?
There is a maximum of 60 object-type fields per object. The Case (case_version__v) object was approaching this limit. This change will ensure that Vault Safety continues to provide the flexibility to add more custom object-type fields going forward.
This change also improves performance for case processing, in areas such as promoting to case, follow-up, page saves, page loads, and more.
What is the Impact?
This update will be seamless to all users. While field types are being replaced, existing data will remain as is, and field system names will remain unchanged. This update will be reflected in the audit log. Finally, configuration elements referencing these fields will also be seamlessly updated. Report generation, including E2B mappings, will not be impacted.
Note Custom triggers or interfaces (not using E2B) that reference Units of Measurement or Reason Omitted fields may require minor changes. Please contact Veeva Managed Services for assistance.
Was this Change Validated?
Yes, this change is validated by Veeva and follows the standard change control process, including PCR and approvals.
Does this Impact All Safety Vaults?
This update will roll out to existing General Release vaults starting May 17, 2021. Limited Release vaults promoted to General Release in April 2021 already have this change.
Affected Fields
Reason Omitted Fields
The following fields, which were previously Reason Omitted object-type fields, are now Picklists.
| Object | Field Name |
|---|---|
|
Case (case_version__v)
|
autopsy_reason_omitted__v |
| dob_reason_omitted__v | |
| dod_reason_omitted__v | |
| gender_reason_omitted__v | |
| last_menstrual_reason_omitted__v | |
| medical_history_text_reason_omitted__v | |
| mrn_gp_reason_omitted__v | |
| mrn_hospital_reason_omitted__v | |
| mrn_investigation_reason_omitted__v | |
| mrn_specialist_reason_omitted__v | |
| patient_id_reason_omitted__v | |
| study_name_reason_omitted__v | |
| study_number_reason_omitted__v | |
| event_onset_reason_omitted__v | |
|
Case Product Dosage (case_product_dosage__v)
|
dose_form_text_reason_omitted__v |
| firstadmin_reason_omitted__v | |
| lastadmin_reason_omitted__v | |
| parent_adminroute_text_reason_omitted__v | |
| patient_adminroute_text_reason_omitted__v | |
| batchlot_number_reason_omitted__v | |
| Case Product Indication (case_product_indication__v) | name_reason_omitted__v |
|
Case Adverse Event (case_adverse_event__v)
|
onset_reason_omitted__v |
| resolved_reason_omitted__v | |
|
Case Study Registration (case_study_registration__v)
|
country_reason_omitted__v |
| registration_number_reason_omitted__v | |
|
Case Contact (case_contact__v)
|
city_reason_omitted__v |
| country_reason_omitted__v | |
| department_reason_omitted__v | |
| firstname_reason_omitted__v | |
| lastname_reason_omitted__v | |
| middlename_reason_omitted__v | |
| organization_reason_omitted__v | |
| postalcode_reason_omitted__v | |
| state_province_reason_omitted__v | |
| street_reason_omitted__v | |
| telephone_reason_omitted__v | |
| title_reason_omitted__v | |
| Case Test Result (case_test_result__v) | date_reason_omitted__v |
|
Case Medical History (case_medical_history__v)
|
continuing_reason_omitted__v |
| enddate_reason_omitted__v | |
| startdate_reason_omitted__v | |
|
Case Drug History (case_drug_history__v)
|
end_date_reason_omitted__v |
| name_reason_omitted__v | |
| startdate_reason_omitted__v |
Units of Measurement Fields
The following fields, which were previously Units of Measurement object-type fields, are now Picklists.
| Object | Field Name |
|---|---|
|
Study Product (study_product__v)
|
dose_unit__v |
| frequency_unit__v | |
| Srudy Product Substance (study_product_substance__v) | strength_unit__v |
|
Study Arm Product (study_arm_product__v)
|
dose_unit__v |
| frequency_unit__v | |
| Product Registration (product_registration__v) | strength_unit__v |
|
Case (case_version__v)
|
age_unit__v |
| gestation_unit__v | |
| height_unit__v | |
| weight_unit__v | |
|
Case Product (case_product__v)
|
cumulative_dose_unit__v |
| gestation_exposure_unit__v | |
| device_age_unit__v | |
|
Case Product Dosage (case_product_dosage__v)
|
dose_unit__v |
| duration_unit__v | |
| frequency_unit__v | |
| Case Product Substance (case_product_substance__v) | strength_unit__v |
| Case Adverse Event (case_adverse_event__v) | duration_unit__v |
|
Case Assessment (case_assessment__v)
|
first_dose_interval_unit__v |
| last_dose_interval_unit__v | |
|
Case Test Result (case_test_result__v)
|
result_qualifier__v |
| result_unit__v |
