Sections in This Article
- About DSUR Reports
- Prerequisites
- Create a DSUR Aggregate Report
- Mark Unexpected Terms in DSUR Reports
- DSUR Table Generation Data Mapping
Note Depending on your Admin's configuration, object, field, and section labels, lifecycle states, and workflows may differ from the general information on this page. Refer to your organization's business processes for guidance.
About DSUR Reports
Vault Safety provides Development Safety Update Report (DSUR) authoring and table generation capabilities, including region-specific table requirements for EMA and FDA submissions. The Vault Safety DSUR report adheres to the ICH E2F regulatory guideline.
The following table summarizes the DSUR tabulations that Vault Safety generates:
| Tabulation | Generated by Default? | Masking Support? |
|---|---|---|
| Cumulative Tabulation of Serious Adverse Events from Clinical Trials | Yes | Yes |
| Interval Line Listings of Serious Adverse Reactions | Yes | Yes |
| Appendix: Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials | No | Yes |
| Appendix: List of Subjects Who Died During the Reporting Period | No | Yes |
Tip An administrator can configure custom DSUR report templates for your organization.
Prerequisites
Consider the following prerequisites before you generate DSUR tables:
- You must be assigned permissions to view and prepare aggregate reports.
Typically, these permissions are reserved for the Safety Writer and Head of Safety roles. - An administrator must have already configured the Reporting Product Family with the Products and Studies to include in the report.
- For vaults originally deployed prior to the 20R2 release (August 2020), to generate DSUR appendices or masked tabulations, an admin must perform feature enablement.
- To generate table data from Study-type Cases, an administrator must have configured the appropriate Study Products.
- Once a study enters the End of Study Reconciliation state for unblinding, each Case must be unblinded prior to table generation. For Studies without Study Arms, you must unblind each suspect product before you can unblind the Case. For Studies with Study Arms, you can directly unblind the Case without having to unblind individual products. Manage Case Blinding provides more information.
- To add a tab at the beginning of the aggregate report document detailing the criteria used to generate the report, your Admin must have enabled the Criteria Page for Aggregate reports.
Create a DSUR Aggregate Report
Create a DSUR Aggregate Report and specify the report settings.
Add a DSUR
- In the vault primary navigation bar, select Aggregate Reports > DSUR, and then select Create.
- In the Create Aggregate Report window, under Select Aggregate Report Type, select DSUR.
- Complete the fields on the Create DSUR page.
- Save the page.
Result
The DSUR record enters the Pending state. The system assigns a task to users in the Safety Writer role to review the report details.
DSUR Fields
You can specify the following fields for a DSUR Aggregate Report:
| Field | Description |
|---|---|
| Product Family (Required) | Select the Reporting Family configured for aggregate reporting. Note The Reporting Family object type should be Product Family. |
| Organization | This field is automatically populated with the Organization on the selected Reporting Family. |
| Data Period Start (Required) | Enter the start date for the reporting period. The system uses the Cases within the reporting period to generate the table data. Cases are included when the date corresponding to the Filter Cases By setting is within the reporting period. Cumulative reports do not consider the start date. The data period contains all Cases up to the Data Period End Date. To learn more, see How Aggregate Reports Filter by Data Period. |
| Data Period End (Required) | Enter the end date for the reporting period. To learn more, see How Aggregate Reports Filter by Data Period. |
| Filter Case By | To customize how the system filter Cases within the specified date range, select an option:
If this field is not specified, the Case Receipt Date/New Info Date are used by default. Depending on when your Vault was originally deployed, an administrator may need to add this field to appear on the page layout. |
| States to Include (Required) | Select the states that Cases must be in to be included in the report. By default, only Cases in the Approved, Closed, Superseded, and Medical Review states are included. Note that while Superseded is not listed as an option, the Closed state includes the Superseded state. Only system-provided states in the Case Processing Lifecycle are supported. Note If the latest Case version within the aggregate reporting period is in the Nullified or Voided state or in a lifecycle state assigned to the Deleted state type, the Case is excluded from the aggregate report. |
| Drug Roles to Include | Select one or more Drug Roles from the dropdown list to include in the following tabulations:
The Suspect and Interacting Drug Roles are selected by default for DSURs created with the 23R3 release. For existing DSURs, update the field to specify or revise which Drug Roles to include in the report. |
| Documents to Generate | You can select which documents to generate. The following options are available:
If you don't specify this field, by default the system generates the following documents:
By default, the documents are unmasked unless you select the Generate Masked Documents option. Depending on when your Vault was originally deployed, an administrator may need to add this field to appear on the page layout. |
| Generate Masked Documents | Select this option to generate a masked copy of the following tables for masked distributions, depending on the tables selected in the Documents to Generate field:
Depending on when your Vault was originally deployed, an administrator may need to add this field to appear on the page layout. To learn more, see Generate Masked Aggregate Tabulations (CIOMS II, PBRER and DSUR). |
| Indicate Unexpected Term | Select Yes to display an asterisk beside each unexpected adverse event term in the following reports:
|
| Datasheet | This field works alongside the Indicate Unexpected Term setting for evaluating approved terms in Product and Study Product Datasheets. For DSUR and PBRER, the system always uses Use Approved Version at the beginning of the reporting period, including when this field is left blank. This setting means that the aggregate report Start Date must be within a term's active range to be considered Expected. To learn more, see Active Range for Expectedness in Aggregate Reports. |
Generate DSUR Tabulations
Review and verify the report settings. Once you have confirmed the report details are correct, use the Generate Aggregate Report Tabulations action to generate DSUR report tables.
Mark Unexpected Terms in DSUR Reports
You can set the Indicate Unexpected Term on a DSUR so that when certain tabulations are generated, the system marks each unexpected adverse event with an asterisk (*).
Unexpected terms can be identified in the following DSUR reports:
- Interval Line Listings of Serious Adverse Reactions (masked and unmasked)
- Appendix: Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials (masked and unmasked)
To identify unexpected events, your Admin must have configured a Datasheet using the following options:
- The Study Product Datasheet for the Study Product that is listed in the DSUR Reporting Family.
- The Core Datasheet for the Study that is listed in the DSUR Reporting Family.
- The Core Datasheet for the primary Investigational Study Product that is listed in the DSUR Reporting Family.
Note Because the DSUR references Datasheets, not Case Assessments, to identify unexpected events, if Indicate Unexpected Term is set to Yes and no Datasheets are configured, all adverse events are marked as unexpected.
The Datasheet can specify the Active Date Start and, optionally, an Active Date End, which indicates when a term is approved as expected for the Product. If configured, The DSUR Start Date must be within a term’s active range to be considered expected.
Active Range for Expectedness in Aggregate Reports provides more information.
DSUR Table Generation Data Mapping
Vault Safety populates aggregate report tables using Cases within the reporting period specified on the DSUR, and the reporting family members configured on the associated Reporting Family.
The following sections describe how Vault Safety generates DSUR tabulations:
- Cumulative Tabulation of Serious Adverse Events from Clinical Trials
- Interval Line Listings of Serious Adverse Reactions
- Appendix: Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials
- Appendix: List of Subjects Who Died During the Reporting Period
Tip For blinded studies, the system populates blinded product information as Blinded in the generated tables.
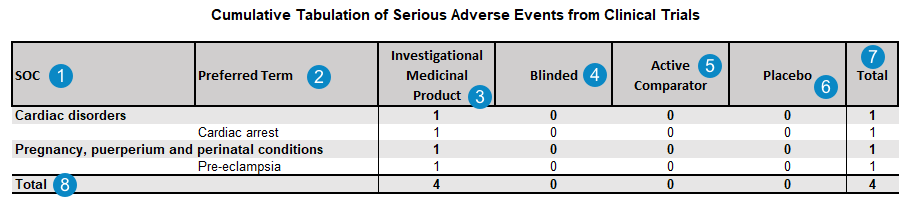
Cumulative Tabulation of Serious Adverse Events From Clinical Trials
The system generates the Cumulative Tabulation of Serious Adverse Events From Clinical Trials by default for DSUR Aggregate Reports.
Note The table above displays the Totals when the DSUR and PBRER Summary Totals and Separate Log Files feature is enabled. Contact your Admin if you would like this feature to be configured your Vault.
Table Constraints
Note In order for a Case to be considered for the report, the Case (created from AER, Inbox Item or Imported Case) must have a Case Product set to Primary.
The system filters Cases to include in the Cumulative Tabulation of Serious Adverse Events From Clinical Trials using the following constraints:
- Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
- Case Report and Study Type
To filter study cases, the system looks at the Case Report Type and Study Type fields. A Case is included when it matches one of the following scenarios:
Scenario Report Type Study Type 1 - Study
- A custom Report Type with an E2B Code of
2
- Clinical Trial
- A custom Study Type with an E2B Code of
1
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version__v.study_product_reason__v.controlled_vocabulary__v.
e2b_code__v = 12 - Study
- A custom Report Type with:
- The E2B Code field set to
2 - The Literature field set to No or Blank
- The E2B Code field set to
- Blank
- A custom Study Type without a valid E2B Code
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version_v.report_type__v.controlled_vocabulary__v.
literature__v ≠ Yes
AND case_version__v.study_product_reason__v = blank - Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the DSUR.
case_version__v.state__v CONTAINS dsur__v.states_to_include__v
Note the following considerations:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
Note You cannot select these states in the States to Include field. These states are always omitted.
- Nullified (
- If the Case is in a Lifecycle State assigned a State Type of "Deleted", the Case is omitted.
- When evaluating the States to Include field, the system evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
- Cases in the following states are omitted:
- Case Date in Cumulative Reporting Period
The date must be within the aggregate report cumulative reporting period (Product IBD to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≤ dsur__v.data_period_end__v
where DATE depends on the option selected in the DSUR Filter Cases By (
dsur__v.filter_cases_by__v) field:- When Approval Date:
case_version__v.approval_date__v
- When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v
- Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, only the most recent Case version within the reporting period is listed.
- When Approval Date:
- Serious Case Adverse Event
The Case Seriousness field contains a value (not blank).
case_version__v.seriousness__v ≠ BLANK
If the Aggregate Report setting Only Include Investigational Products for SAR is enabled, the report only considers Adverse Events where the Case Assessment Causality Established field is set to either Yes or blank (unknown) for any Study Product on the Case. The Study Product must be Blinded (blank) or has a Study Product Role of Investigational, Placebo, or Active Comparator.
Note Your Admin must first enable this Aggregate Report setting in your Vault.
- Study Member of Reporting Family
The Study field links to a Study record that is either:
- A member of the Reporting Family
case_version__v.study__v CONTAINS
reporting_family__v.reporting_family_member__v.study__v - Contains a Study Product that matches a Product Reporting Family Member
case_version__v.study__v CONTAINS
reporting_family__v.reporting_family_member__v.products__v.study_product__v.study__v
- A member of the Reporting Family
Note To ensure Blind Protection, unblinded Cases are counted as blinded until End of Study Reconciliation unblinding is complete for each Case.
Table Mapping
The following table outlines how the system maps data to populate the Cumulative Tabulation of Serious Adverse Events From Clinical Trials:
| Number | Name | Description |
|---|---|---|
| SOC | The MedDRA System Organ Class (SOC) for the adverse event.
case_adverse_event__v.event_meddra__v.soc_term__v |
|
| Preferred Term | The MedDRA Preferred Term (PT) for each adverse event, grouped by the MedDRA SOC.
case_adverse_event__v.event_meddra__v.pt_term__v Note Contact Veeva Support to request PT Aggregation in periodic reports, which counts only unique instances of Preferred Terms (PT) in summary tabulations. Once this feature is enabled, when a Case contains multiple Case Adverse Events coded under the same MedDRA Preferred Term (PT), the report counts a single PT event instead of multiple events. |
|
| Investigational Medicinal Product | The total number of adverse events with suspect investigational products.
COUNT IF
|
|
| Blinded | The total number of adverse events with suspect blinded products.
COUNT IF
|
|
| Active Comparator | The total number of adverse events with suspect active comparators.
COUNT IF
|
|
| Placebo | The total number of adverse events with suspect placebos.
COUNT IF
|
|
| Total | The sum of the Investigational Medicinal Product, Blinded, Active Comparator, and Placebo SAE occurrences for each SOC and Preferred Term. | |
| Total | The total number of SAE occurrences for each of the Investigational Medicinal Product, Blinded, Active Comparator, and Placebo categories. |
If the DSUR and PBRER Summary Totals and Separate Log Files feature is enabled in your Vault, the Cases in the report are listed in a separate log file once the system generates the report.
If the DSUR and PBRER Summary Totals and Separate Log Files feature is not enabled in your Vault, the Cases in the report are listed in a separate table as part of the report:
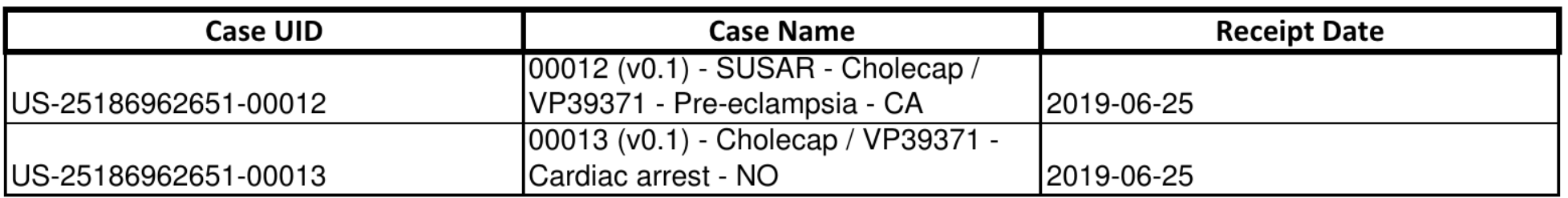
The Investigational Medicinal Products (IMP) table contains a breakdown of the Products that appear in the Investigational Medicinal Products column in the main table.
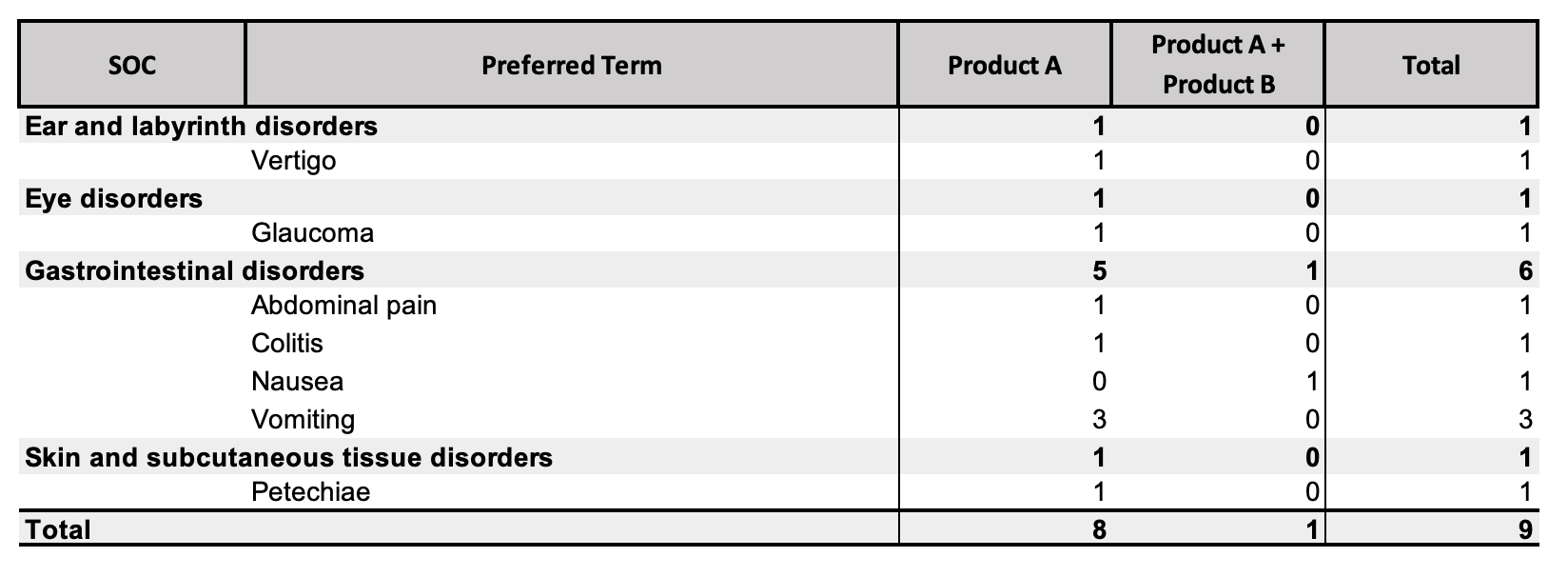
Note The table above displays the Totals when the DSUR and PBRER Summary Totals and Separate Log Files feature is enabled. Contact your Admin if you would like this feature to be configured your Vault.
The IMP breakdown table considers only Cases that contain Investigational Product roles and Products that are in the Reporting Family. The IMP breakdown table contains a column for each combination of Products that appear in the Cases included on the main report. Up to a maximum of ten (10) combinations are supported. The sum of these totals corresponds with the total Adverse Event count for the Investigational Medicinal Product on the main report.
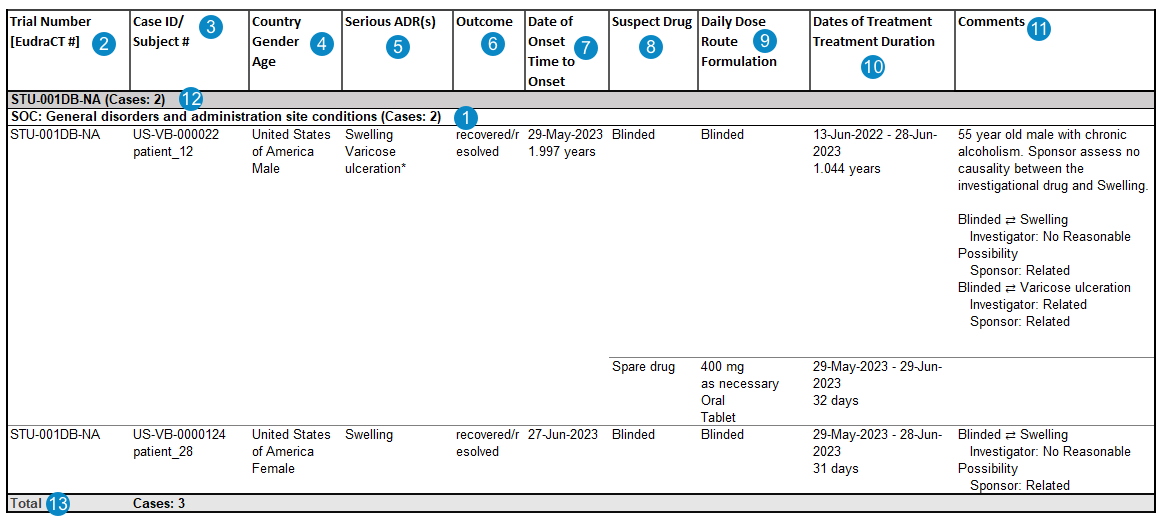
Interval Line Listings of Serious Adverse Reactions
The system generates the Serious Adverse Reactions from Clinical Trials Line Listings by default for all DSUR Aggregate Reports.
Table Constraints
The system filters Cases to include in the Interval Line Listings of Serious Adverse Reactions using the following constraints:
- Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
- Study Member of Reporting Family
The Study field links to a Study record that is either:
- A member of the Reporting Family
case_version__v.study__v CONTAINS
reporting_family__v.reporting_family_member__v.study__v - Contains a Study Product that matches a Product Reporting Family Member
case_version__v.study__v CONTAINS
reporting_family__v.reporting_family_member__v.products__v.study_product__v.study__v
- A member of the Reporting Family
- Case Report and Study Type
To filter study cases, the system looks at the Case Report Type and Study Type fields. A Case is included when it matches one of the following scenarios:
Scenario Report Type Study Type 1 - Study
- A custom Report Type with an E2B Code of
2
- Clinical Trial
- A custom Study Type with an E2B Code of
1
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version__v.study_product_reason__v.controlled_vocabulary__v.
e2b_code__v = 12 - Study
- A custom Report Type with:
- The E2B Code field set to
2 - The Literature field set to No or Blank
- The E2B Code field set to
- Blank
- A custom Study Type without a valid E2B Code
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version_v.report_type__v.controlled_vocabulary__v.
literature__v ≠ Yes
AND case_version__v.study_product_reason__v = blank - Case Date in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≥ dsur__v.data_period_start__v AND
DATE ≤ dsur__v.data_period_end__vwhere DATE depends on the option selected in the DSUR Filter Cases By (
dsur__v.filter_cases_by__v) field:- When Approval Date:
case_version__v.approval_date__v
- When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v
- Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, only the most recent Case version within the reporting period is listed.
- When Approval Date:
- Serious Case Adverse Event
The Case Seriousness field contains a value (not blank).
case_version__v.seriousness__v ≠ BLANK
If the Aggregate Report setting Only Include Investigational Products for SAR is enabled, the report only considers Adverse Events where the Case Assessment Causality Established field is set to either Yes or blank (unknown) for any Study Product on the Case. The Study Product must be Blinded (blank) or has a Study Product Role of Investigational, Placebo, or Active Comparator.
Note Your Admin must first enable this Aggregate Report setting in your Vault.
- Causality Established is Yes or Blank on Any Case Assessment
The Causality Established field must be either Yes or blank (unknown) on any Case Assessment to consider the Case.
case_assessment_result.causality_established == (Yes OR Blank)A Case is excluded from this report if all serious Case Adverse Events are assessed as unrelated. That is, if all serious Case Adverse Events are linked with at least two Case Assessment Results with the Causality Established field set to No, where:
- One Case Assessment Result is for the company ("Sponsor" or "MAH"). That is, the Source Type maps to E2B Code
2or4. - One other Case Assessment Result where the Source Type does not map to E2B Code
2or4.
If the Aggregate Report setting Only Include Investigational Products for SAR is enabled, the report only considers Cases where the Study Product is either Blinded (blank) or has a Study Product Role of Investigational, Placebo, or Active Comparator.
Note Your Admin must first enable this Aggregate Report setting in your Vault.
- One Case Assessment Result is for the company ("Sponsor" or "MAH"). That is, the Source Type maps to E2B Code
- Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the DSUR.
case_version__v.state__v CONTAINS dsur__v.states_to_include__v
Note the following considerations:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
Note You cannot select these states in the States to Include field. These states are always omitted.
- Nullified (
- If the Case is in a Lifecycle State assigned a State Type of "Deleted", the Case is omitted.
- When evaluating the States to Include field, the system evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
- Cases in the following states are omitted:
- Case Product in Drug Roles to Include
Only include Case Products in the listing where the Drug Role matches one of the Drug Roles specified on the Drug Roles to Include field on the DSUR.
case_product__v.drug_role__v CONTAINS dsur__v.drug_roles_to_include__v
Table Mapping
The following table outlines how the system maps data to populate the Interval Line Listings of Serious Adverse Reactions:
| Number | Name | Description |
|---|---|---|
| SOC & Case Total |
The MedDRA System Organ Class (SOC) for the adverse event. case_adverse_event__v.event_meddra__v.soc_term__v The total number of unique Cases listed under the SOC for the Trial is shown in parentheses. |
|
| Trial Number [EudraCT#] | For studies registered to a country in the European Union, values are mapped from the following fields:
|
|
| Case ID/ Subject # | Values from the following fields:
|
|
| Country, Gender, Age | Values from the following fields:
|
|
| Serious ADR(s) |
The MedDRA Preferred Term for the serious adverse event. case_adverse_event__v.event_meddra__v.pt_term__v
The primary adverse event is listed first. |
|
| Outcome | The value selected in the Case Adverse Event Outcome field. If there are multiple Case Adverse Event records on a Case, the system populates the most serious outcome, per E2B guidelines.
case_adverse_event__v.event_outcomes__v.name__v
|
|
| Date of Onset, Time to Onset | Values are mapped for the primary Case Adverse Event as follows:
|
|
| Suspect Drug |
The name of the primary Case Product. While the product is blinded, the value is Blinded. If the product is unblinded, the unblinded version of the Interval Line Listings displays the name of the primary Case Product. On the blinded version of the Interval Line Listings, the value remains Blinded. First row (Primary Case Product):
IF [case_version__v.case_product__v.primary__v = Yes
Subsequent rows (non-Primary Case Products):
IF [case_version__v.case_product__v.primary__v != Yes
|
|
| Daily Dose, Route, Formulation |
If the primary Case Product is blinded, the value is Blinded. First row (Primary Case Product):
IF (case_product__v.primary__v = Yes)
Subsequent rows (non-Primary Case Products):
IF [case_version__v.case_product__v.primary__v != Yes
If the primary Case Product is not blinded, values are mapped from the primary Case Product > Case Product Dosage as follows:
If there are multiple Dosages under the primary Case Product, values from each Dosage record are displayed in a line-separated list. |
|
| Dates of Treatment, Treatment Duration |
Values are mapped from the primary Case Product > Case Product Dosage object as follows:
|
|
| Comments |
Values from the following fields:
|
|
| Trial Number & Case Total |
The Trial Number only (excluding the EudraCT#). See row The total number of distinct Cases listed under the Trial in the report is shown in parentheses. |
|
| Total | The total number of distinct Cases across all Trials in the report. |
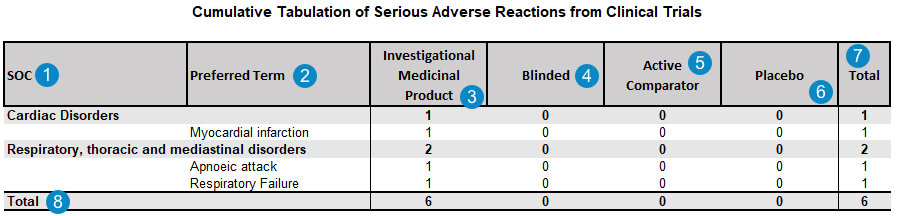
Appendix: Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials
To generate the Cumulative Summary Tabulation of Serious Adverse Reactions table for a DSUR report, select this table in the Documents to Generate field on the DSUR Aggregate Report record.
In vaults originally deployed before the 20R2 release (August 2020), an administrator must Enable DSUR Appendices Generation.
Note The table above displays the Totals when the DSUR and PBRER Summary Totals and Separate Log Files feature is enabled. Contact your Admin if you would like this feature to be configured your Vault.
Note The Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials appendix tabulation does not list data about treatment arms or identify unexpected adverse reactions.
Table Constraints
Note In order for a Case to be considered for the report, the Case (created from AER, Inbox Item or Imported Case) must have a Case Product set to Primary.
The system filters Cases to include in the Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials using the following constraints:
- Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
- Case Report and Study Type
To filter study cases, the system looks at the Case Report Type and Study Type fields. A Case is included when it matches one of the following scenarios:
Scenario Report Type Study Type 1 - Study
- A custom Report Type with an E2B Code of
2
- Clinical Trial
- A custom Study Type with an E2B Code of
1
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version__v.study_product_reason__v.controlled_vocabulary__v.
e2b_code__v = 12 - Study
- A custom Report Type with:
- The E2B Code field set to
2 - The Literature field set to No or Blank
- The E2B Code field set to
- Blank
- A custom Study Type without a valid E2B Code
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version_v.report_type__v.controlled_vocabulary__v.
literature__v ≠ Yes
AND case_version__v.study_product_reason__v = blank - Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the DSUR.
case_version__v.state__v CONTAINS dsur__v.states_to_include__v
Note the following considerations:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
Note You cannot select these states in the States to Include field. These states are always omitted.
- Nullified (
- If the Case is in a Lifecycle State assigned a State Type of "Deleted", the Case is omitted.
- When evaluating the States to Include field, the system evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
- Cases in the following states are omitted:
- Case Date in Cumulative Reporting Period
The date must be within the aggregate report cumulative reporting period (Product IBD to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≤ dsur__v.data_period_end__v
where DATE depends on the option selected in the DSUR Filter Cases By (
dsur__v.filter_cases_by__v) field:- When Approval Date:
case_version__v.approval_date__v
- When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v
- Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, only the most recent Case version within the reporting period is listed.
- When Approval Date:
- Serious Case Adverse Event
The Case Seriousness field contains a value (not blank).
case_version__v.seriousness__v ≠ BLANK
If the Aggregate Report setting Only Include Investigational Products for SAR is enabled, the report only considers Adverse Events where the Case Assessment Causality Established field is set to either Yes or blank (unknown) for any Study Product on the Case. The Study Product must be Blinded (blank) or has a Study Product Role of Investigational, Placebo, or Active Comparator.
Note Your Admin must first enable this Aggregate Report setting in your Vault.
- Causality Established is Yes or Blank on Any Case Assessment
The Causality Established field must be either Yes or blank (unknown) on any Case Assessment to consider the Case.
case_assessment_result.causality_established = (Yes OR Blank)If the Aggregate Report setting Only Include Investigational Products for SAR is enabled, the report only considers Cases where the Study Product is either Blinded (blank) or has a Study Product Role of Investigational, Placebo, or Active Comparator.
Note Your Admin must first enable this Aggregate Report setting in your Vault.
- Study or Substance Reporting Family Member
One of the following conditions must be met:
- The Study field links to a Study record that is either:
- A member of the Reporting Family
OR - Contains a Study Product for a Product that is a member of the Reporting Family
- A member of the Reporting Family
- A Case Product links to a preconfigured Substance that is a member of the Reporting Family.
case_version__vr.study__v CONTAINS
(reporting_family__vr.reporting_family_member__vr.products__vr.study_product__vr.study__v
OR reporting_family__vr.reporting_family_member__vr.study__v)
OR
WHERE reporting_family_v.substance__v.substance_v is not BLANK
AND reporting_family_v.substance__v.substance_v = case_product__v.product__v.product_substance__v
AND case_version__v.report_type__v = study - The Study field links to a Study record that is either:
Table Mapping
The following table describes how Vault Safety generates the Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials:
| Number | Report Field | Logic |
|---|---|---|
| SOC | The MedDRA System Organ Class (SOC) for the adverse event.
case_adverse_event__v.event_meddra__v.soc_term__v |
|
| Preferred Term | The MedDRA Preferred Term (PT) for the adverse event. All qualifying Case Adverse Events are listed by MedDRA PT, grouped by the MedDRA SOC. case_adverse_event__v.event_meddra__v.pt_term__v The system evaluates each Case Adverse Event relatedness for each Study Product before listing the event in this report. If at least one Study Product is assessed as related to the Case Adverse Event (or blank/unknown), the event is listed. Note Contact Veeva Support to request PT Aggregation in periodic reports, which counts only unique instances of Preferred Terms (PT) in summary tabulations. Once this feature is enabled, when a Case contains multiple Case Adverse Events coded under the same MedDRA Preferred Term (PT), the report counts a single PT event instead of multiple events. A Case Adverse Event is excluded from this report when two or more Case Assessment Results for the event and study product have Causality Established set to No, where:
EXCLUDE IF: |
|
| Investigational Medicinal Product | The total number of adverse events with a primary suspect Study Case Product and the Study Product Role field set to Investigational on the associated Study Product record.
COUNT IF
|
|
| Blinded | The total number of adverse events with a blinded primary suspect Study Case Product.
COUNT IF:
|
|
| Active Comparator | The total number of adverse events with a primary suspect Study Case Product and the Study Product Role field set to Active Comparator on the associated Study Product record.
COUNT IF
|
|
| Placebo | The total number of adverse events with a primary suspect Study Case Product and the Study Product Role field set to Placebo on the associated Study Product record.
COUNT IF
|
|
| Total | The sum of the Investigational Medicinal Product, Blinded, Active Comparator, and Placebo SAR occurrences for each SOC and Preferred Term. | |
| Total | The total number of SAR occurrences for each of the Investigational Medicinal Product, Blinded, Active Comparator, and Placebo categories. |
If the DSUR and PBRER Summary Totals and Separate Log Files feature is enabled in your Vault, the Cases in the report are listed in a separate log file once the system generates the report.
If the DSUR and PBRER Summary Totals and Separate Log Files feature is not enabled in your Vault, the Cases in the report are listed in a separate table as part of the report:

The Investigational Medicinal Products (IMP) table contains a breakdown of the Products that appear in the Investigational Medicinal Products column in the main table.

Note The table above displays the Totals when the DSUR and PBRER Summary Totals and Separate Log Files feature is enabled. Contact your Admin if you would like this feature to be configured your Vault.
The IMP breakdown table considers only Cases that contain Investigational Product roles and Products that are in the Reporting Family. The IMP breakdown table contains a column for each combination of Products that appear in the Cases included on the main report. Up to a maximum of ten (10) combinations are supported. The sum of these totals corresponds with the total Adverse Event count for the Investigational Medicinal Product on the main report.
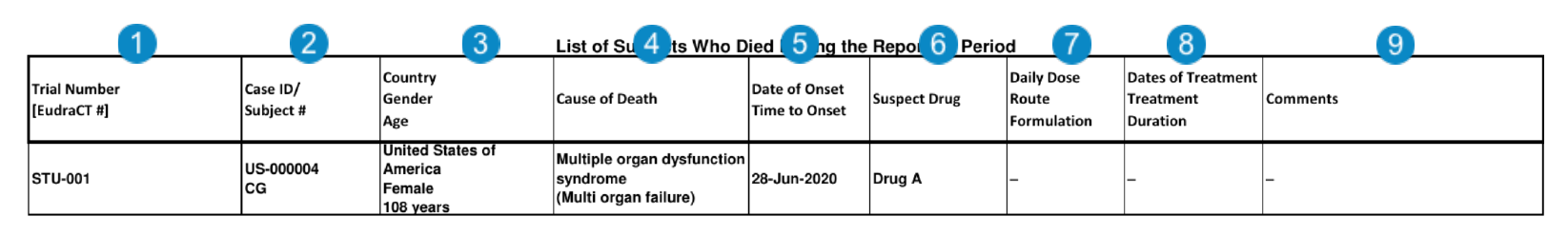
Appendix: List of Subjects Who Died During the Reporting Period
To generate the List of Subjects Who Died During the Reporting Period table for a DSUR report, select this table in the Documents to Generate field on the DSUR Aggregate Report record.
In vaults originally deployed before the 20R2 release (August 2020), an administrator must Enable DSUR Appendices Generation.
Table Constraints
The following list describes how the system filters Cases to include the List of Subjects Who Died During the Reporting Period.
- Case Not Suppressed
The Case Suppress Submission field must be set to No or blank (not suppressed).
case_version__v.suppress_submission__v ≠ Yes
- Case Report and Study Type
To filter study cases, the system looks at the Case Report Type and Study Type fields. A Case is included when it matches one of the following scenarios:
Scenario Report Type Study Type 1 - Study
- A custom Report Type with an E2B Code of
2
- Clinical Trial
- A custom Study Type with an E2B Code of
1
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version__v.study_product_reason__v.controlled_vocabulary__v.
e2b_code__v = 12 - Study
- A custom Report Type with:
- The E2B Code field set to
2 - The Literature field set to No or Blank
- The E2B Code field set to
- Blank
- A custom Study Type without a valid E2B Code
case_version_v.report_type__v.controlled_vocabulary__v.
e2b_code__v = 2
AND case_version_v.report_type__v.controlled_vocabulary__v.
literature__v ≠ Yes
AND case_version__v.study_product_reason__v = blank - Study Member of Reporting Family
The Study field links to a Study record that is either:
- A member of the Reporting Family
case_version__v.study__v CONTAINS
reporting_family__v.reporting_family_member__v.study__v - Contains a Study Product that matches a Product Reporting Family Member
case_version__v.study__v CONTAINS
reporting_family__v.reporting_family_member__v.products__v.study_product__v.study__v
- A member of the Reporting Family
- Case Lifecycle State in Aggregate States to Include
The latest Case version within the reporting period must be in a state specified in the States to Include field on the DSUR.
case_version__v.state__v CONTAINS dsur__v.states_to_include__v
Note the following considerations:
- Cases in the following states are omitted:
- Nullified (
nullified_state__v) - Voided (
voided_state__v)
Note You cannot select these states in the States to Include field. These states are always omitted.
- Nullified (
- If the Case is in a Lifecycle State assigned a State Type of "Deleted", the Case is omitted.
- When evaluating the States to Include field, the system evaluates Cases in the Superseded (
superseded_state__v) state as Closed (closed_state__v).
- Cases in the following states are omitted:
- Case Indicates a Death Occurred
One of the following conditions must be met to indicate a death occurred:
- A value in the Date of Death field
case_version__v.dod_normalized__v ≠ Blank
- The Case Seriousness field contains Results in Death
case_version__v.seriousness__v = results_in_death__v
- Any Case Adverse Event Outcome field contains Fatal
case_version__v.case_adverse_event__v.outcome__v = fatal
- Any Case Adverse Event has a coded event under the MedDRA HLT of 10011907 (Death and Sudden Death).
case_adverse_event__v.event_meddra__v.meddra__v.hlt_code__v = 10011907
- A value in the Case Autopsy field.
case_version__v.autopsy_value__v = ≠ Blank
- The Case contains a Case Cause of Death record.
case_version__v.case_cause_of_death__v ≠ 0
- A value in the Date of Death field
- Case Date in Interval Reporting Period
The date must be within the aggregate report interval reporting period (Data Period Start to Data Period End). How Aggregate Reports Filter by Data Period provides more information.
DATE ≥ dsur__v.data_period_start__v AND
DATE ≤ dsur__v.data_period_end__vwhere DATE depends on the option selected in the DSUR Filter Cases By (
dsur__v.filter_cases_by__v) field:- When Approval Date:
case_version__v.approval_date__v
- When blank or Receipt Date / New Info Date (Default):
- If the Case New Info Date (
new_info_date__v) is blank, the Receipt Date is used:case_version__v.receipt_date__v
- Otherwise, the New Info Date is used:
case_version__v.new_info_date__v
- If the Case New Info Date (
If there are multiple versions of the Case within the reporting period, only the most recent Case version within the reporting period is listed.
- When Approval Date:
- Case Product in Drug Roles to Include
Only include Case Products in the listing where the Drug Role matches one of the Drug Roles specified on the Drug Roles to Include field on the DSUR.
case_product__v.drug_role__v CONTAINS dsur__v.drug_roles_to_include__v
Table Mapping
The following table describes how Vault Safety generates the List of Subjects Who Died During the Reporting Period:
| Number | Name | Description |
|---|---|---|
| Trial Number [EudraCT#] |
For studies registered to a country in the European Union, values are mapped from the following fields:
|
|
| Case ID/ Subject # |
Values from the following fields:
|
|
| Country Gender Age |
Values from the following fields:
|
|
| Cause of Death | The MedDRA preferred term (PT) associated with each Case Cause of Death record, followed by the reported (verbatim) term enclosed in brackets.
case_cause_of_death__v.cause_of_death_meddra_pt__c
|
|
| Date of Onset Time to Onset |
Values are mapped for the primary Case Adverse Event as follows:
|
|
| Suspect Drug |
The name of the primary Case Product. If the product is blinded, the value is Blinded. To ensure Blind Protection, unblinded Cases are counted as blinded until End of Study Reconciliation unblinding is complete for each Case. First row (Primary Case Product):
IF [case_version__v.case_product__v.primary__v = Yes
Subsequent rows (non-Primary Case Products):
IF [case_version__v.case_product__v.primary__v != Yes
|
|
| Daily Dose Route Formulation |
If the primary Case Product is blinded, the value is Blinded. First row (Primary Case Product):
IF (case_product__v.primary__v = Yes)
Subsequent rows (non-Primary Case Products):
IF [case_version__v.case_product__v.primary__v != Yes
If the primary Case Product is not blinded, values are mapped from the primary Case Product > Case Product Dosage as follows:
If there are multiple Dosages under the primary Case Product, values from each Dosage record are displayed in a line-separated list. |
|
| Dates of Treatment Treatment Duration |
Values are mapped from the primary Case Product Dosages as follows:
|
|
| Comments |
Any text entered in the Case Reporting Summary field.
case_version__v.reporting_summary__v |