Sections in This Article
About Schema Validation
Vault Safety validates E2B files against the XSD (XML Schema Definition) to catch E2B errors prior to ICSR submissions. The XML is validated automatically as part of the E2B generation process.
During validation, the E2B file is validated against the appropriate agency’s schema. The following list describes how the system validates each type of E2B file type:
- EMA E2B(R3) are validated against the EMA XSD schema
- FDA E2B(R2) files are validated against FDA ICSR XML DTD version 2.1
- FDA E2B(R2) files with combination products are validated against FDA ICSR XML DTD version 2.2
- HC E2B(R2) files are validated against FDA ICSR XML DTD version 2.1
- FDA VAERS E2B(R3) files are validated against the EMA XSD schema
- PMDA E2B(R3) files are validated against PMDA XSD schema
About Case and Submission Business Validation
Vault Safety performs validation for E2B(R2) and (R3) formats (ICH/EMA/FDA/FDA VAERS/PMDA). E2B Schema validation occurs at the Submission level once the E2B file is generated. Business validation occurs at the Case and Transmission levels (Submission and Distribution).
Non-SUSAR and manual submissions are not Case validated because Case validation operates on rule engine evaluation. Transmissions created manually are validated but the associated Case is not validated. Transmissions must reference a Transmission Profile, for a supported file format, for the file to be validated. For example, if the Outbound Format field on the Transmission Profile is set to Other, no Validation Results records are generated because the format does not require validations.
Note Consider the following limitations for E2B validation on Distribution-type Transmissions:
- If the Always Evaluate field on the Validation Criteria object is not set to Yes, Vault Safety does not validate E2B XML files generated for Email Distributions to regional destinations.
- Other Distributions (non-email) are validated, but the system does not send a notification when validation is complete.
- Vault Safety does not support Case-level validation for Imported Cases. See Migrate External Cases for more information on Imported Cases.
Custom Validation Criteria
If required, your Admin can configure your Vault to add Validation Criteria that are not already included in Vault Safety. See Configure Custom Validation Criteria for more information.
FDA Character Encoding Validation
The FDA supports only ISO 8859-1 characters for E2B(R2) submissions and does not support the UTF-8 character set.
During FDA E2B(R2) report validation at the Case and Transmission level, if any text fields or narrative documents have non-ISO 8859-1 characters, the system generates a validation warning along with a CSV file to capture the validation results for conformance with ISO 8859-1. The CSV file provides a direct link to the validation error for the character. This is a separate file from the validation file, which captures all other validation errors.
ISO Smart Replace
Some UTF-8 characters with a matching character in ISO 8859-1 are auto-replaced in the generated FDA E2B(R2) file, while the remainder are replaced with blank fields.
The following table displays the UTF-8 characters that are auto-replaced with ISO 8859-1-compliant characters:
| UTF-8 Character | ISO 8859-1-Compliant Character |
|---|---|
| “ | " |
| ” | " |
| ‘ | ' |
| ’ | ' |
| – | - |
| — | - |
| Other non-ISO-compliant character | [White Space] |
Configure the Excel File to View Unsupported Characters
When you open the ISO 8859-1 validation CSV file with Microsoft Excel, the Unsupported Characters column contains ambiguous characters because Excel does not automatically encode them as UTF-8.
To view these characters in the CSV file, perform the following steps:
- Close the downloaded file.
- Open a new Excel file.
- Perform one of the following actions depending on your operating system:
- Mac OS: Go to File > Import.
- Windows: Go to Data > From Text/CSV.
- Select CSV file then select Import.
- In your folder, select the downloaded CSV file and then select Get Data.
- From the File Origin dropdown, select Unicode (UTF-8), and then select Next.
- Under Delimiters, deselect Tab, and then select Comma.
- Perform one of the following actions depending on your operating system:
- Mac OS: Select Finish.
- Windows: Select Load.
Result
The unsupported characters on the CSV file are now viewable when you open the file.
Business Validation
Business validation occurs at the Case and/or Transmission level. Validations are completed based on global (ICH) and regional (EMA, FDA VAERS, PMDA) conformance rules. Regional validation occurs only at the Submission and Distribution levels.
- Over 190 ICH conformance rules validated at the Case and Transmission level against E2B(R3) documents (this includes ICH E2B(R3), EMA E2B(R3), and FDA VAERS E2B(R3) file formats).
- Over 170 FDA VAERS conformance rules validated at the Transmission level against FDA VAERS E2B(R3) file format.
- Over 100 EMA conformance rules validated at the Transmission level against EMA E2B(R3) file format.
- Over 150 PMDA conformance rules validated at the Case and Transmission level against the PMDA E2B(R3) file format.
Some regional rules supersede (override) ICH global rules. In this case, only the regional rule is validated at the Transmission level.
Vaults created in 20R2 or earlier must be configured to add Case-level validation and to add the Validation Results section to Submission and Distribution page layouts.
Business validation results with fail or warning outcomes are reported in the Validation Results object. If you regenerate the E2B file, the reported validation results are deleted and new validation results records are created if there are any failures or warnings specific to the validation criteria.
Trigger Validation
The following describes when the system performs validation:
- Transmissions: When a Submission or Distribution file is created, Vault automatically validates against the schema (for supported file formats), and against the Global (ICH) and regional (FDA VAERS, EMA, or PMDA) validation criteria.
- Cases: A Case is validated when a user triggers the Evaluate Regulatory Conformance action. Admins can configure your Vault to automatically run the Evaluate Regulatory Conformance action as part of the Case lifecycle.
Business validation results with fail or warning outcomes are reported in the Validation Results object. If you regenerate the E2B file, the reported validation results are deleted and new validation results records are created if there are any failures or warnings, specific to the validation criteria.
Note Regional validations are not executed on E2B formats that will not be sent to a region.
View Validation Results
When a validation criteria rule evaluates as a fail or warning, a corresponding Validation Result record is created for the Case and Transmission. Validation Result records can be viewed from a Case or Transmission record. The Validation Results Summary record is also created for each validated Case and Transmission. The Validation Results Summary record is listed with the other Validation Results (including regional FDA VAERS, PMDA, and EMA E2B(R3) validation results). Each Validation Results Summary record includes an attachment detailing all the results of the rules run: pass, fail, and warning.
The sections below describe the Validation Criteria and Validation Result objects. Validation Criteria and Validation Results appear on Cases or Transmissions after validation.
Note Validation Rules and Results are system managed. You cannot modify or delete existing system rules or validation rules.
Validation File
Upon validation completion, the system generates a CSV file that captures the pass or fail result of each validation rule.
The file is added as an attachment to the Transmission (Submission or Distribution) record and linked to from Vault notifications.
Validation Criteria Fields
The Validation Criteria object captures validation rule criteria (such as business and conformance rules) based on industry guidance.
The following table describes the fields on the Validation Criteria object:
| Field | Description |
|---|---|
| Agency | Agency the rule originated from. For global (i.e. ICH) rules, this field will be blank. |
| API Name | The record's unique identifier. |
| Conformance | Business, conformance, schema or other rule definition based on the Source. |
| Data Element | The Data Element number, as specified in the guidance document, for the evaluated value. |
| Data Element Name | The name of the Data Element, as specified in the guidance document. |
| Evaluation Level | The level at which the rule should evaluate (i.e. Case or Submission). |
| File Format | The format used to drive validation rules. |
| Result Status Type | Select Fail, Warning, or Hard Fail as the The rule result outcome. This field also populates the Validation Status on the Case or Transmission.
Note the difference between a Fail and Hard Fail value:
|
| Rule Formula | The formula used to validate the data element conformance. |
| Rule Formula Format |
The format used for formula execution. This includes:
If left blank, the system defaults to File Format. Note File Format is used for all standard ICH E2B(R3) validation rules. |
| Rule Version | The validation criteria version. |
| Always Evaluate | If selected, the system evaluates this Validation Rule for all global and regional destinations regardless of the Case having a reporting obligation. |
| Source | The origin of the validation rule. |
| Vault Field | The name of the field to be evaluated. |
| Vault Object | The name of the object to be evaluated. |
| Supersedes | Select a rule to override with the new conformance. The Superseded rule will not evaluate for the parameters specified. |
| Superseded Conformance | The conformance for the superseded rule. |
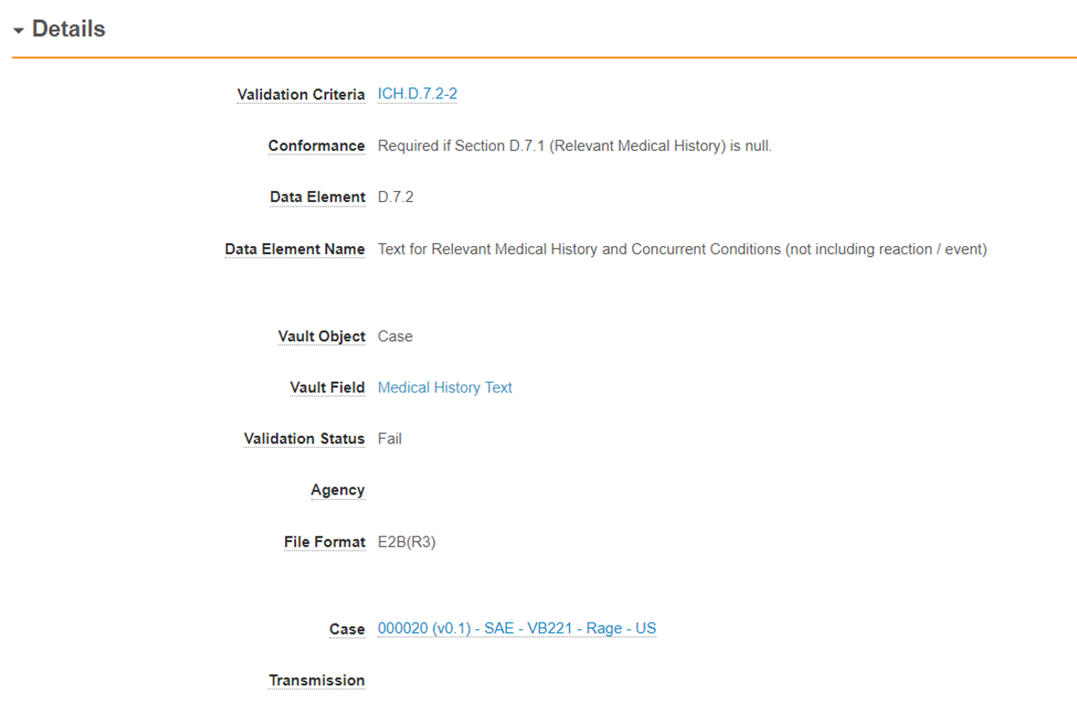
Validation Result Fields
The Validation Result object captures validation criteria results from the imported Case or Submission. Validation Results indicate failed and warning messages - rules that pass validation are not displayed.
Note Not all warnings are displayed as validation results. Previously implemented warnings will continue to be sent via notifications or email.
The following table describes the fields on the Validation Result object:
| Field | Description |
|---|---|
| Validation Criteria | Reference to the validation criteria that resulted in the rule evaluation. |
| Conformance | Reference to the validation criteria conformance that resulted in the rule evaluation. Value is inherited from the Validation Criteria. |
| Data Element | The Data Element number, as specified in the guidance document, for the evaluated value. |
| Data Element Name | The name of the Data Element, as specified in the guidance document. |
| Vault Object | The name of the object to be evaluated. |
| Vault Field | The name of the field to be evaluated. |
| Validation Status | Indicates the rule outcome: pass, fail, or warning. |
| Agency | Agency the rule originated from. For global (i.e. ICH) rules, this field will be blank. |
| File Format | The format used to drive validation rules. |
| Case | The Case record this validation result was evaluated for. |
| Transmission | Specifies when the validation occurs at the Transmission level. This field references the related Transmission. This field will be blank if the rule was evaluated at the Case level. |
| Validation Criteria Version | Version of the Validation Criteria used for the evaluation. |
| Organization | Name of the organization associated with the Case. |
| Vault Record ID | This is the record id for the evaluated field. We recommend you remove this field from page layouts. |
View All Validation Criteria
Your Admin can access a complete list of validation criteria in your Vault by navigating to Business Admin > Validation Criteria.
Tip To download a list of the validation criteria from the Business Admin > Validation Criteria page, expand the All Actions menu, and then select Excel or CSV.
Ignore Validation Results
When a validation criteria rule evaluates as a fail or warning, you can ignore the validation result for the given criteria for any subsequent evaluations of that Case or Transmission. This option allows for Submissions without deleting any Case data, even when the evaluation returns validation failures, which helps to ensure submissions are not blocked.
Prerequisites
Your Admin must perform the configurations in Enable Ignore Validation Rule to use this feature.
Ignore Validation Results for a Case
- Go to Cases and select the Case you want to submit.
- In the Validation Results (Failures & Warnings) section, select the Actions menu next to any validation results you want to ignore.
- Select Ignore Validation Result. You can include this validation result in evaluations again by selecting Activate Validation Result.
Result
The system ignores the respective validation results for the Case and its associated Transmissions. The Case can now be submitted, considering all other criteria are met. The summary record will not include the validation result.
Ignore Validation Results for a Transmission
- Go to Transmissions > Distributions or Submissions.
- Select a Transmission.
- In the Validation Results (Failures & Warnings) section, select
 next to any validation results you want to ignore.
next to any validation results you want to ignore. - Select Ignore Validation Result. You can include this validation result in evaluations again by selecting Activate Validation Result.
Result
The system ignores the respective validation results for that Transmission only. The summary record will not include the validation result.
Assign Hard Fail to Validation Rules
We recommend that your Admin configure the following Result Status Types to be “Hard Fail”:
- ICH.C.1.3-1
- ICH.C.1.3-2
- ICH.C.1.4-1
- ICH.C.1.4-2
- ICH.C.1.4-3
- ICH.C.1.5-1
- ICH.C.1.5-2
- ICH.C.1.5-3
- ICH.C.2.r.3-1
- ICH.C.2.r.3-2
- ICH.C.5.4-1
- ICH.C.5.4-2
- ICH.D.1
- ICH.E.i.4-1
- ICH.E.i.4-2
- ICH.E.i.7-1
- ICH.E.i.7-2
- ICH.G.k.1-1
- ICH.G.k.1-2
- ICH.G.k.2.2
This is a recommended list. You may choose to add more or less of these depending on your business’s evaluation of severity of each validation criteria.
- Go to Business Admin > Objects > Validation Criteria.
- Select [Validation Criteria].
- Select Hard Fail from the Result Status Type field.
- Select Save.
Your Admin must also configure workflow transitions for Case validations.
Data Entry Validation
In addition to validating E2B XML during file generation, Vault Safety validates certain fields during Case data entry to prevent issues that would generate an invalid E2B file.
Value and Unit Field Validation
The system validates all standard (system-provided) combined Value and Unit fields.
When a field contains invalid data, the system prevents you from saving the record and marks the field with an Invalid label.
See the following example, where the Weight field has a unit but no value:

Reason Omitted Field Validation
The system validates standard (system-provided) Reason Omitted (nullFlavour) fields, with the exception of those listed below. Reason Omitted fields allow you to select only valid NullFlavour values, according to E2B specifications.
Data Entry Validation Limitations
Consider the following limitations for data entry validation:
- Custom (non-standard) fields are not validated.
- The following Reason Omitted (nullFlavour) fields are not validated or filtered to valid values:
Object or Document Type Field Case Contact Object Country (reason omitted) ( country_reason_omitted__v)Case Object Medical History Text (reason omitted) ( medical_history_text_reason_omitted__v)Note To comply with E2B specifications, ensure Case page layouts include the Medical History Text app control-type field, which is filtered to valid values for Reasons Omitted. For more information see Update the Case Page Layout for Reasons Omitted for Medical History Text.
Case Object Study Name (reason omitted) ( study_name_reason_omitted__v)Case > Source > Literature Document Type Reference (reason omitted) ( reference_reason_omitted__v)
Prerequisites for E2B XML Validation
While E2B XML validation is automatically available in all vaults, we recommend that an administrator performs the following configuration to prevent the Submit to Gateway action when the system detects a validation error.
-
Read More...
- In Admin, go to Configuration > Object Lifecycles > Transmission Lifecycle.
- Open the Validation Error lifecycle state.
- Under Atomic Security: Actions, select Edit.
- For the Submit to Gateway action, change the State Behavior to Hide.
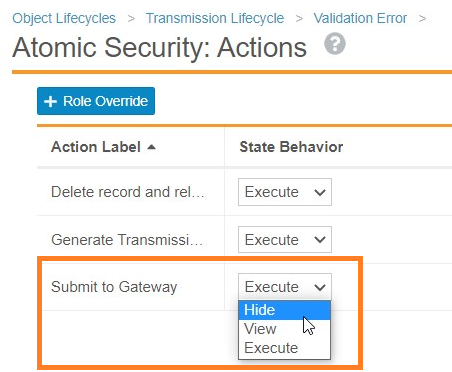
Change Submit to Gateway Behavior to Hide
Trigger E2B XML Validation
To validate an E2B file, generate an EMA E2B(R3), FDA E2B(R2), or HC E2B(R2) file from a Transmission (Submission or Distribution) record.
The system validates files generated using either of the following methods:
- Manually with the Generate Transmission Document(s) user action.
- Automatically as part of a Submission or Distribution workflow.
Note The system does not validate E2B files generated from the Case.
Notifications
The system notifies the appropriate user if the system found errors during validation with a Vault notification and email. These notifications are sent in addition to notifications about the success or failure of generating the transmission file.
The following list describes which users receive notifications:
- If the file was generated manually with a user action, the system notifies the user who started that action.
- If the file was generated automatically through a workflow, the system notifies Distribution Manager users for the Transmission Organization.
- Otherwise, the system notifies the System Admin user group.
Resolve Validation Errors
Once the Submission record enters the Pending state, the system generates an E2B XML file and immediately validates it against the corresponding schema. At the same time, the Review Submission workflow starts, and the Distribution Manager should accept their task as they do in their standard workflow.
If there are validation errors, the Submission record lifecycle state changes to Validation Error. The Distribution Manager must review and fix the validation errors attached in the CSV file. Once the errors are resolved, the Distribution Manager must regenerate the transmission documents.
If there are no further validation errors, the system removes the attached validation CSV file and you can proceed with completing the Submission workflow.
If there are new validation errors, the appropriate user receives a notification and a validation CSV file replaces the previous attachment.
E2B Error Message Guide
The following tables describe the E2B validation errors you may encounter, and the possible cause of each error message.
EMA E2B(R3) Error Messages
| Error | Possible Cause |
|---|---|
| cvc-complex-type.2.4.a: Invalid content was found starting with element 'responseModeCode'. One of '{urn:hl7-org:v3:creationTime}' is expected. | The Submission record is missing a value in the Transmission Date field. |
| cvc-complex-type.2.4.a: Invalid content was found starting with element 'creationTime'. One of '{urn:hl7-org:v3:realmCode, urn:hl7-org:v3:typeId, urn:hl7-org:v3:templateId, urn:hl7-org:v3:id}' is expected. | The Submission record is missing a value in the E2B Message ID field. |
| cvc-complex-type.2.4.a: Invalid content was found starting with element 'controlActProcess'. One of '{urn:hl7-org:v3:sequenceNumber, urn:hl7-org:v3:attachmentText, urn:hl7-org:v3:receiver}' is expected. | A Transmission Profile was not found. Either the Transmission Profile was not configured, or it was not assigned to the Submission record correctly. Verify the Transmission Profile field on the Submission record links to a Transmission Profile, and verify the record is set up correctly. |
| cvc-complex-type.2.4.b: The content of element 'MCCI_IN200100UV01' is not complete. One of '{urn:hl7-org:v3:PORR_IN049016UV, urn:hl7-org:v3:PORR_IN049017UV, urn:hl7-org:v3:PORR_IN049018UV, urn:hl7-org:v3:receiver}' is expected. | A Transmission Profile was not found. Either the Transmission Profile was not configured, or it was not assigned to the Submission record correctly. Verify the Transmission Profile field on the Submission record links to a Transmission Profile, and verify the record is set up correctly. |
FDA and HC E2B(R2) Error Messages
| Error | Possible Cause |
|---|---|
| The content of element type ichicsrmessageheader must match (messagetype, messageformatversion, messageformatrelease, messagenumb, messagesenderidentifier, messagereceiveridentifier, messagedateformat, messagedate). | The message header is not formatted correctly due to missing Submission details. For example, the Submission record may be missing values in the Transmission Date or E2B Message ID fields. |
