Sections in This Article
Localized Business Administration
Vault Safety supports capturing data in fields required by regional agencies, such as the PMDA. Your Admin can preconfigure certain data in the Business Admin library.
In Vaults originally deployed in 20R3 or earlier, you must enable localized submissions and translation support before configuring localized Business Admin records.
Set Up Localized Product and Substance Information
Add a Japan Product Registration
- Go to Business Admin > Objects > Products and create the Product.
If the Product is already in your Vault, go to the record. - Under the Product, add a Product Registration child record.
- Set the Product Registration Country to Japan.
- Use the PMDA section to enter Japan product registration data.
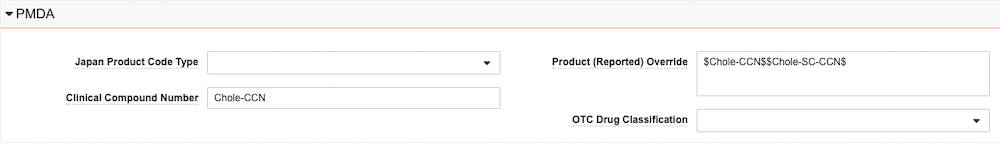
Product Registration Japan Fields
Japan Product Registration Fields
| Field | Description |
|---|---|
| Registration Type | Select the product Registration Type. |
| Dose Form | Select the dose form. |
| Japan Product Code Type | Select the classification of the local product code for Japan. |
| Local Product Code | Enter the region-specific product code: J-Drug Code, OTC, or Temporal. |
| Clinical Compound Number | Enter the Japanese Clinical Compound Number (CCN) of the investigational drug. When reporting Study Cases for Clinical Trial Studies registered in Japan, enter the CCN on the Study Registration record to export that value to the J2.12 Clinical Compound Number (CCN) data element of PMDA E2B(R3) reports. |
| Product (Reported) Override | Enter a value to map to Case Product Registration records on Cases where Case Products are registered to Japan for foreign Cases, such as where the Case Reporter Country is not set to Japan. This value is exported to the G.k.2.2 Medicinal Product Name as Reported by the Primary Source data element on PMDA E2B(R3) reports. This field supports the scenario when a foreign Case Product has multiple investigational registrations with studies conducted in Japan with the same Substance. |
| OTC Drug Classification | Select the PMDA OTC Drug Risk category. |
Add a Localized Substance
- Go to Business Admin > Objects > Products and open the Product.
- Add the Substance under the Product.
If the Substance is already in your vault, open the Substance. - Under the Substance, add a Localized Substance child record.
You may need to update the Substance page layout to add the Localized Substance related object section.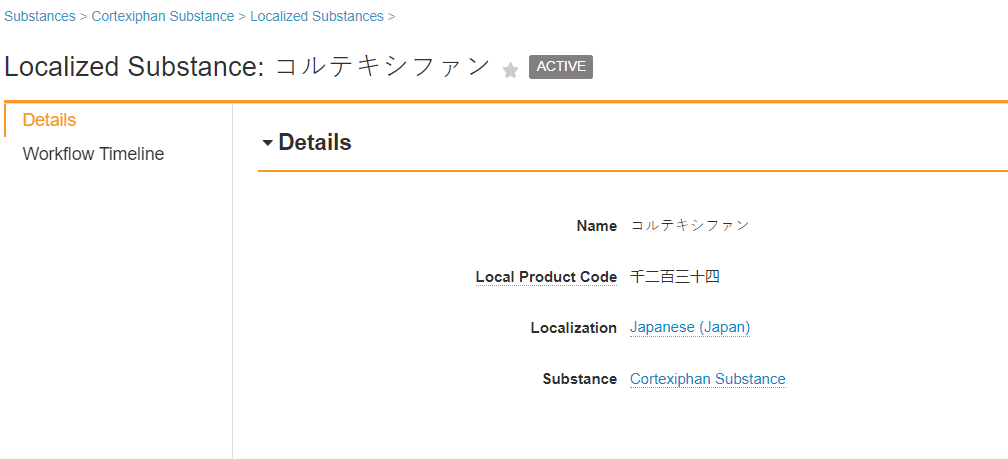
Japan Localized Substance
Japan Substance Fields
| Field | Description |
|---|---|
| Name | Enter the Japanese substance name. |
| Local Product Code | Enter the Japan local product code. |
| Localization | Select the locale for the regional data. |
| Substance | The referenced Substance record. The system automatically populates this field when you create the Localized Substance as a child object from the initial Substance. |
Add a Japan Dose Form Code
You can configure the 3-letter PMDA E2B Code for Dose Forms users can select during PMDA data entry.
- Go to Business Admin > Objects > Dose Form and open the Dose Form.
Under the Dose Form, you can see a Localized Dose Form. - Open the Localized Dose Form child record.
- Set the value of E2B Code.
Set Up Localized Study Information
Add Localized Study information to automatically populate PMDA information for Localized Study Cases.
Add the Study
- Go to Business Admin > Objects > Studies and create the Study.
If the Study is already in your Vault, go to the record. - Specify the Development Phase field on the Study for PMDA submissions:
Field Description Development Phase Select the development phase of the study. 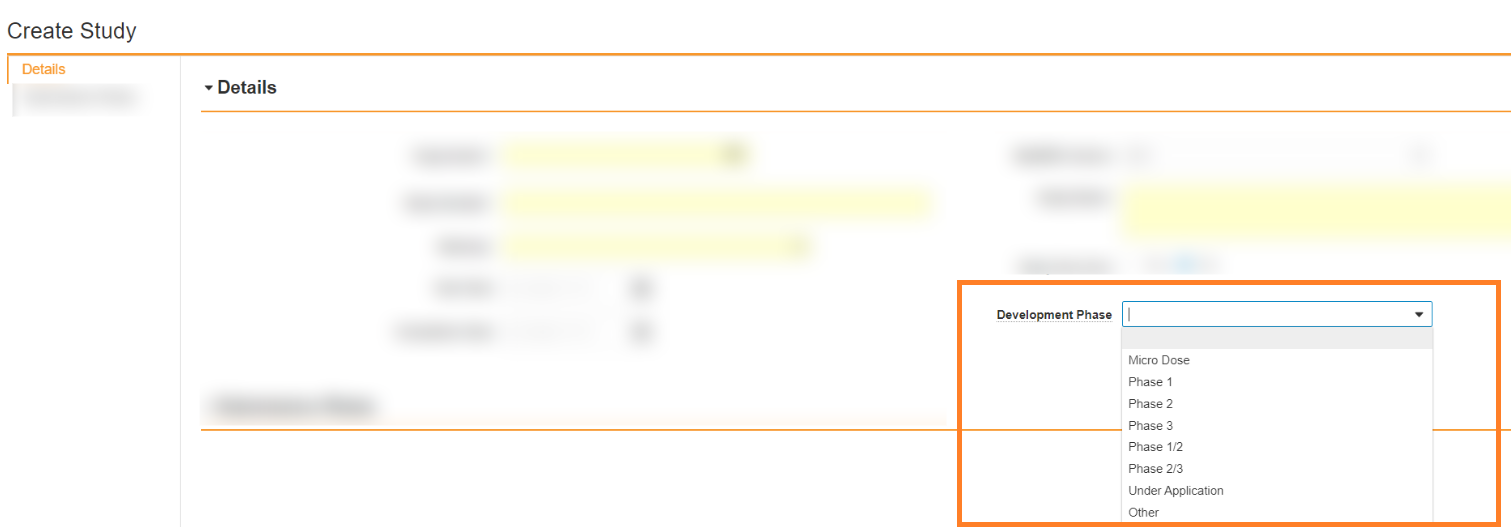
Development Japan Field
Add a Localized Study
- Open the Study.
- Under the Study, add a Localized Study child record.
You may need to update the Study page layout to add the Localized Study related object section.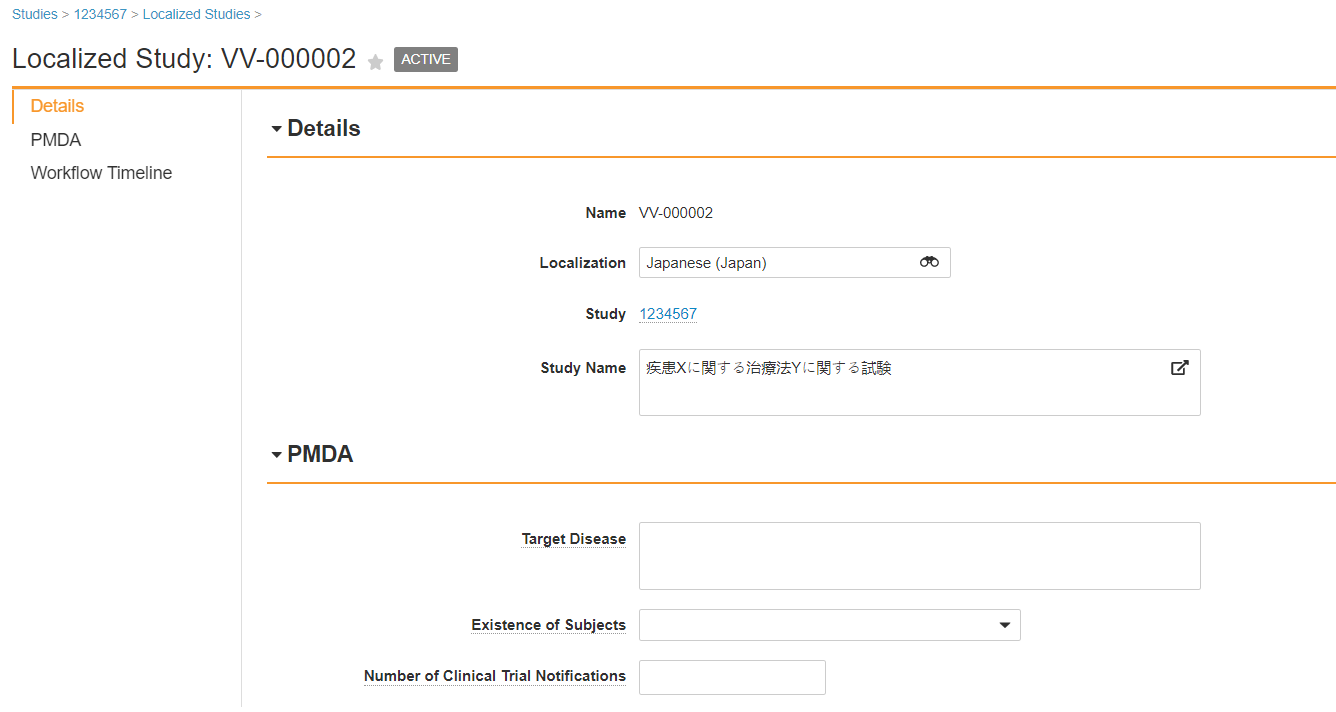
Japan Localized Study
Japan Localized Study Fields
| Section > Field | Description |
|---|---|
| Registrations > Clinical Compound Number | Enter the Japanese Clinical Compound Number (CCN) of the investigational drug. This field appears only if the Country field is set to Japan. When reporting Study Cases for Clinical Trial Studies registered in Japan, the system populates this value in the J2.12 Clinical Compound Number (CCN) data element of PMDA E2B(R3) reports. If this field is blank, the system maps the CCN of the primary Case Product Registration from the Local Reporting Details section of the Case. This field must be configured by your Admin to appear on page layouts. For more information, see Enable PMDA Clinical Trial Reporting Enhancements. |
| Localizations > Localization | Select Japanese (Japan). |
| Localizations > Study | The referenced Study record. The system automatically populates this field if you create the Localized Study as a child object from the initial Study. |
| Localizations > Study Name | Enter the Japanese study name. |
| Localizations > Target Disease | Describe the disease being studied. |
| Localizations > Existence of Subjects | Select Yes or No, to indicate whether subjects are present in this study. |
| Localizations > Number of Clinical Trial Notifications | Enter the number of clinical trial notifications submitted to the PMDA for this study. |
Create Japan Organizations (Non-PMDA Only)
Vault Safety includes Japan regulatory agency PMDA out of box. If you prepare submissions for the PMDA only, you can skip these steps. For other Japan reporting destinations, such as Partner Distributions, create an Organization.
- Go to Business Admin > Objects > Organizations and create the Organization.
If the Organization is already in your vault, go to the record. - On the Organization, set the Localization field to Japan.
You may need to update the Organization page layout to add this field. - Under the Organization, add a Localized Organization child record.
You may need to update the Organization page layout to add the Localized Organization related object section. - Set up reporting rules for the destination organization.
You must set up reporting rules to automatically generate Localized Japan Cases when reporting obligations are evaluated.
Set Up Localizations
You can configure Localization records to meet specific reporting needs and reduce manual effort when working on Domestic and Localized Cases. For example, the Japan Localization can be configured with the following behaviors:
- Auto-population of reportable Case Product Registrations to expedite data entry for the J2 data elements of PMDA E2B(R3) reports.
- Generate Localized Case Assessments and Expectedness for all Case Product Registrations on the Localized Case. This feature offers the option to calculate Due Dates at the Transmission level or for each Localized Case Assessment.
Prerequisites
For reporting to the PMDA, your Admin must configure the following Localization record features:
- Enable PMDA Local Case Processing Automations for retrieving reportable Case Product Registrations and configuring Assessment generation.
- Enable PMDA Due Dates Based on Localized Case Assessments for setting which date field is used to calculate Due Dates.
These features support evaluating the most reportable Case Assessment to ensure compliance with PMDA reporting requirements.
Manage Localizations
To manage Localization records, go to Business Admin > Objects > Localizations. When editing a record, reference the table below when populating fields.
| Field | Description |
|---|---|
| Localization Type | The available Localization Types are Global and Local. This impacts whether Inbox Items and Cases are processed as Global, Domestic, or Localized. Your Admin can configure your Vault to Auto-select Inbox Item Localization by Reporter Country. |
| Localization Scope | This field manages which fields are available for dual-language Domestic Case processing. Leave this field blank for the Localization Scope to include all fields. Otherwise, you can set the scope to only Narratives, Reporter Comments, or both. If configured, the localized values for Reporter Comments and Narratives are also exported in E2B formats. |
| Assessment Generation | This field supports Localized Case Assessment generation based on Japanese Case Product Registrations only when reporting to the PMDA. Select how Case Assessments are generated, as follows:
|
| Localized Due Date Calculation | When the Assessment Generation field is set to Localized Assessments for Case Product Registrations, you can select the method for calculating Due Dates on Localized Cases for Japan as follows:
|
