Safety supports dual language intake for entering cases that are received in a non-English language but require reporting in English. This article covers this intake scenario only. For localized (non-English) submissions, see Prepare a Localized Case.
When you promote an Inbox Item with local data to a Case, Vault generates a Case in the local language. Vault maps the relevant field values to the Cases.
For example, the EMA requires ICSR submissions in English. If an adverse event that occurred in Germany is reported in German. Intake users can enter data in the source language (German) while entering the corresponding English translations in the text fields on the Inbox Item. When the Inbox Item is promoted to a Case, Vault maps the data to create a Global Case which can be used for English Submissions.
Note: When processing Cases that may be reportable to the PMDA, see Complete Intake and Process Cases for the PMDA for regionally specific features in Vault Safety.
Prerequisites
- For Vaults originally deployed in 22R1 (April 2022) and earlier, your Admin must add the Default Localization field to the User Profile.
- For Vaults originally deployed in 21R1 (April 2021) or earlier, your Admin must Enable Local Language to English Intake.
- If using the Auto-Translation Framework for local language to English translations, your Admin must enable the Auto-Translation Framework.
Send Translation Requests Through the Auto-Translation Framework
If your organization has set up the Auto-Translation Framework, after manually creating or importing a local Inbox Item, you can send all localized text fields to Amazon Translate for translation to English.
For imported Cases, although Inbox Items do not display all Case fields, all localized text fields with values are sent for translation, unless excluded by your Admin’s configuration. When Inbox Items are created manually, only those fields that support dual-language entry are sent for translation. For more information on supported fields, see Text Field Mapping and Translation on Case Promotion.
When translations are returned, you can review text fields on the Inbox Item or after Case promotion.
Note: Narratives and documents are not currently supported through the Auto-Translation Framework.
Translation requests must be sent manually after Inbox Item creation.
Select the All Actions menu, and then select Translate Inbox Item.
After Vault sends the translation request, the Inbox Item enters the Translation Requested state.
When third-party translations are generated and returned to Vault, they are automatically applied to the Inbox Item. Vault does not verify the quality of the translation, but simply imports it. The Inbox Item enters the Translation Verification state.
Considerations for Translation Requests
- Inbox Items in the Translation Requested state are editable. If text fields are edited in this state, the updated values are overridden when translations are returned to Vault.
- If the length of the translated text exceeds the character limit of any text field, the text is truncated to that field’s maximum length. Vault displays a notification message indicating which fields have been truncated.
- The translation must be returned within 60 minutes or the task will expire. Vault displays a notification message for failed translation requests.
Translation Notifications
Depending on your Admin’s configuration, Vault may send translation notification messages in the following scenarios:
- When a translation error occurs, Vault notifies the user who sent the Inbox Item for translation and moves the Inbox Item to the Translation Error state. A translation error can occur if the translation is not returned to Vault within 60 minutes or if the translation service goes offline during the translation.
- When a translation is complete and pending verification, Vault notifies the user who sent the Inbox Item for translation.
Display Localized Field Labels
You can view fields in Vault in your preferred language by selecting a language from your User Profile. When you change this setting, all field labels and picklist values in Vault will display in the selected language. The Default Localization field determines the language in Object Reference fields, such as Report Type.
Note: If the Default Localization field is blank, Vault automatically refers to the Language field to determine the language for the picklist field values.
Configuring the above settings allows you to perform localized manual intake with Inbox Items.
Show Dual Entry Fields
When an Inbox Item has a a non-global Localization, you can perform local language to English translation. To include local intake fields:
- In the Localization field, select the language the adverse event was reported in.
The Localization field is typically in the Organization and Region section. - Select Save.
For Localizations with a Localization Scope limited to Narratives and/or Company Comments, dual-entry fields appear only after you promote an Inbox Item to a Case.
Local Intake Fields with English Translations
The following sections provide more information on how information is translated during intake:
For descriptions of Inbox Item fields see, Inbox Item Field Reference.
Translate Text Fields
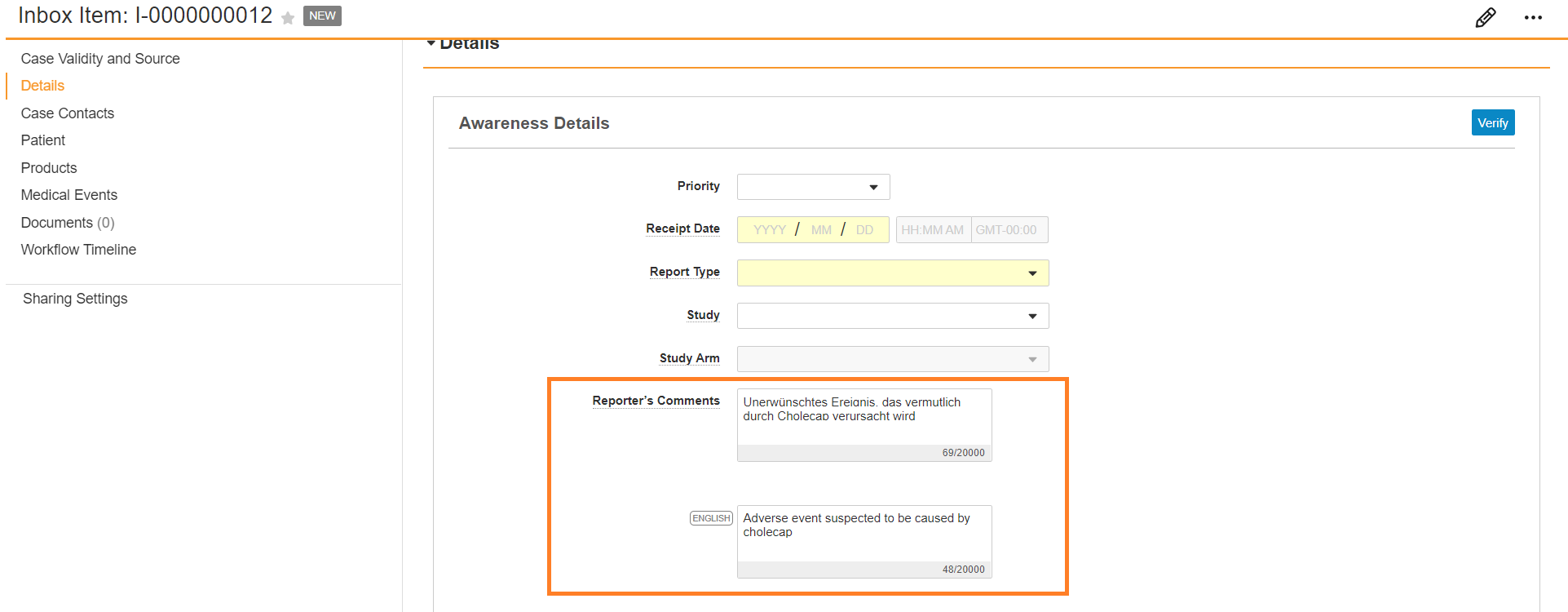
You can manually translate text fields during intake. For each text field that supports dual language intake, there are two fields:
- In the first field, enter the information in the language the adverse event was reported in.
- In the second field, marked with
 , enter the English translation.
, enter the English translation.
Text Field Mapping and Translation on Case Promotion
On Case promotion, only the  field translations are mapped to the Global Case. The field values in the local language are not copied over to the Global Case.
field translations are mapped to the Global Case. The field values in the local language are not copied over to the Global Case.
Visible Inbox Item Fields
The following table shows Inbox Item fields that are available for translation and describes how Vault maps text fields with local and English values on Case promotion:
| Inbox Item | Case | ||
|---|---|---|---|
| Section |  Field Field |
Object | Field |
| Details | Reporter Comments | Case (Narrative) | Reporters Comments |
| Case Contact | Additional Information | Case Contact > Details | Additional Information |
| Case Contact | Organization | Case Contact > Details | Organization |
| Case Contact | Department | Case Contact > Details | Department |
| Case Contact | State/Province | Case Contact > Address | State/Province |
| Product | Product (Reported) | Case Product | Product (Reported) |
| Product | Indication (Reported) | Case Product | Indication |
| Product > Dosage | Dose Form (Custom)* | Case Product Dosage | Dose Form |
| Product > Dosage | Patient RoA (Custom)* | Case Product Dosage | Patient RoA |
| Product > Dosage | Dose Text | Case Product Dosage | Dose Text |
| Drug History | Drug (Reported) | Drug History | Drug (Reported) |
| Medical Events | Event (Reported) | Adverse Event | Event (Reported) |
| *Text translations are only available when entering custom Dose Forms or Patient RoAs. Translations for standard system-provided Dose Forms and Patient RoA values are automatically mapped upon Case promotion. | |||
Imported Case Text Fields Eligible for Auto-Translation
Review the table below for all importable Case text fields that may be included in Translation Requests. While not all of these fields are displayed or editable on Inbox Items, all translated English values are passed through to the Case after promotion.
| Field | Field Labels | ||
|---|---|---|---|
| Case > Medical History Text | (medical_history_text__v) |
||
| Case > Reporter's Comments | (reporters_comments__v) |
||
| Case > Company Comments | (sender_comments__v) |
||
| Case > CIOMS Remarks | (cioms_remarks__v) |
||
| Case > Reporting Summary | (reporting_summary__v) |
||
| Case > Study Name | (study_name__v) |
||
| Case > Study Name (continued) | (study_name_continued__v) |
||
| Case Adverse Event > Event (Reported) | (event_reported__v) |
||
| Case Assessment Result > Method (Text) | (method_text__v) |
||
| Case Assessment Result > Result (Text) | (result_text__v) |
||
| Case Assessment Result > Source (Text) | (source_text__v) |
||
| Case Cause of Death > Name (Reported) | (name_reported__v) |
||
| Case Contact > Additional Information | (additional_information__v) |
||
| Case Contact > City | (city_value__v) |
||
| Case Contact > Department | (department_value__v) |
||
| Case Contact > Facility | (facility_name__v) |
||
| Case Contact > First Name | (firstname_value__v) |
||
| Case Contact > Last Name | (lastname_value__v) |
||
| Case Contact > Middle Name | (middlename_value__v) |
||
| Case Contact > Organization | (organization_value__v) |
||
| Case Contact > State / Province | (state_province_value__v) |
||
| Case Contact > Street | (street_value__v) |
||
| Case Contact > Street Line 2 | (street_line_2_value__v) |
||
| Case Contact > Title | (title_value__v) |
||
| Case Drug History > Drug (Reported) | (name_reported__v) |
||
| Case Drug History > Drug (Coded) | (product_name__v) |
||
| Case Identifier > Source* | (source__v) |
||
| Case Medical History > Condition / Procedure (Reported) | (name_reported__v) |
||
| Case Medical History > Comments | (comments__v) |
||
| Case Product > Other Additional Information | (additional_information_text__v) |
||
| Case Product > Product (Reported) | (product_reported__v) |
||
| Case Product Dosage > Dose Text | (dose_text__v) |
||
| Case Product Dosage > Dose (Unit Text) | (dose_unit_text__v) |
||
| Case Product Dosage > Parent RoA Text | (parent_adminroute_text__v) |
||
| Case Product Dosage > Patient RoA Text | (patient_adminroute_text__v) |
||
| Case Product Indication > Name (Reported) | (name_reported__v) |
||
| Case Product Substance > Name | (name__v) |
||
| Case Case Test Result > Comments | (comments__v) |
||
| Case Case Test Result > Result (Text) | (result_text__v) |
||
| Case Case Test Result > Test Name (Reported) | (name_reported__v) |
||
| *When imported through E2B, the Source value is not available for translation. | |||
Picklist and Object Field Translations
Any system-provided and managed picklists and object lookup fields are shown in the value selected in the Default Localization field on your User Profile. Note that custom picklists and object records will not be automatically translated:
- Controlled Vocabularies
- Route of Administrations
- Dose Forms
- Units of Measurement
- Reasons Omitted
Event MedDRA Translations
The Event field on Adverse Events includes MedDRA details that may automatically translate coded MedDRA terms in the applicable language. For more information about how to code multilingual MedDRA terms, see Code MedDRA Terms.