Note As of 20R2, the MedDRA auto-code control is automatically deployed and replaces the legacy MedDRA fields. This enablement page is deprecated and will be removed in a future release.
About the Feature
MedDRA auto-coding was added in Vault Safety 20R1. Upgraded vaults must perform the following configuration changes to enable this feature.
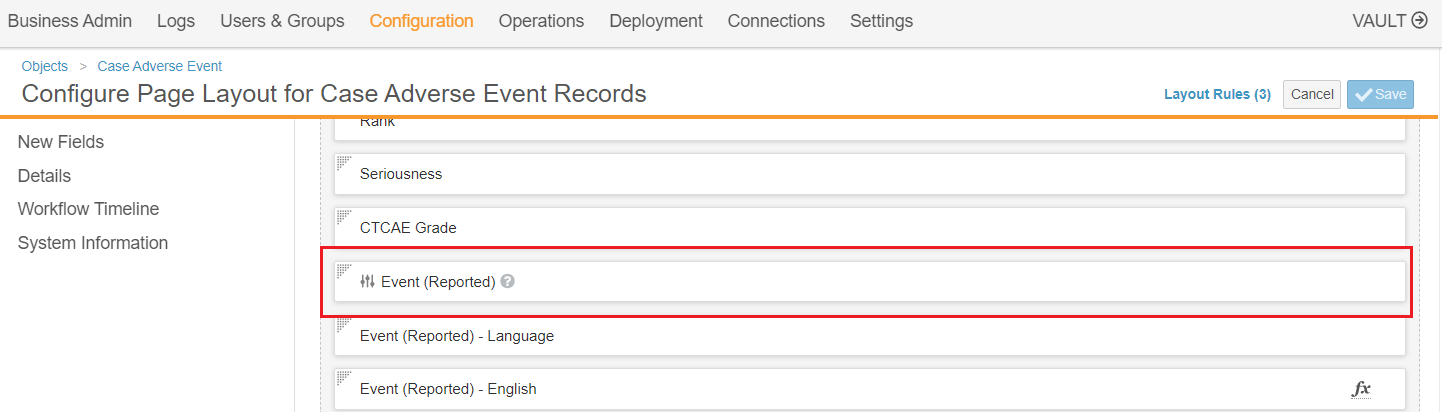
To support MedDRA auto-coding, this feature adds a new combined MedDRA control to replace the previously separate reported term and MedDRA term fields. You must add this field to page layouts to enable this feature.
The new control allows users to automatically code MedDRA terms using the reported term. The following image shows the combined MedDRA control before entering a term:

The following image shows the MedDRA control after auto-coding a term:
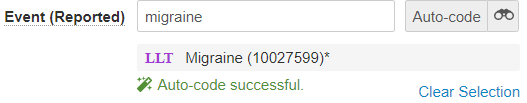
Code MedDRA Terms provides more information about coding with the MedDRA dictionary.
Add the MedDRA Control to Page Layouts
To configure object page layouts, go to Configuration > Objects, and then on the appropriate object, open the Page Layouts tab.
Note The MedDRA auto-code control is not available on the Case Diagnosis or Case Drug History objects.
| Object | Page Layouts | Change |
|---|---|---|
| Case | AER Detail Page Layout |
|
| Case Product |
|
To enable the auto-code control for Case Product Indications, you must add the Indications section control to Case Product page layouts. Enable Case Product Section Controls provides instructions. |
| Case Adverse Event | Case Adverse Event Detail Page Layout |
|
| Case Test Result | Case Test Result Detail Page Layout |
|
| Case Medical History | Case Medical History Detail Page Layout |
|
| Case Cause of Death |
|
|
The following image shows the MedDRA control added to a page layout: