Vault determines whether a Case should be tagged as a Designated Medical Event (DME) or with a preconfigured watchlist.
Note: Depending on your Admin’s configuration, your Vault’s object, field, and section labels, lifecycle states, and workflows may differ from the general information in all articles. Refer to your organization’s business processes for guidance.
About Watchlist Tags and DMEs
You can maintain watchlists for Adverse Events of Special Interest (AESIs) and other Important Medical Events (IMEs) that require monitoring. Vault can tag Cases for one or more watchlists, which can be specific to or independent of marketed Products or Studies. For Studies with Unspecified Products, Vault applies Watchlist Tags to Case Products with an eligible Drug Role on Cases for that Study.
All Safety Vaults include an up-to-date official DME watchlist. By default, the DME watchlist monitors only Cases with Case Products or Study Products referencing a Product registered in a country under the jurisdiction of the European Medicines Agency (EMA). Your Admin can configure additional adverse event watchlists.
Depending on your Admin’s configuration, Cases may be expedited when they are tagged for certain watchlists, regardless of expectedness or causality.
Default Case Seriousness
Your Admin can configure watchlists to assign a default Seriousnesss to an adverse event. Doing so will automatically assign the Case Seriousness for Inbox Items containing the respective adverse event after promoting it to a Case.
Note: If the Inbox Item being promoted already has a specified Seriousness (it is not blank), the watchlist will not update the Case Seriousness upon Case promotion.
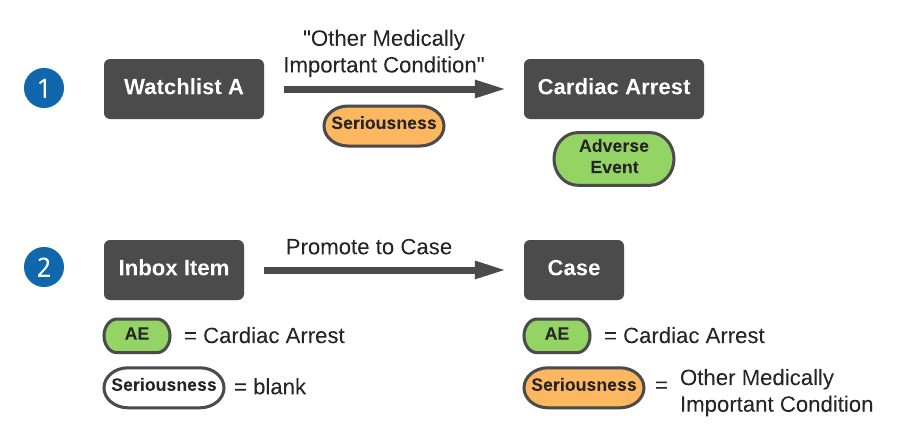
The following diagram describes an example of a watchlist assigning Case Seriousness to an Inbox Item with an unspecified (blank) Seriousness field:
- Watchlist A sets the Seriousness to “Other Medically Important Condition” for adverse event Cardiac Arrest.
- After you promote an Inbox Item containing adverse event Cardiac Arrest and a blank Seriousness field to a Case, the Case Seriousness will be automatically set to “Other Medically Important Condition”.
If multiple watchlists are configured to set the Case Seriousness for the same adverse event, Vault will assign all specified Seriousness criteria to the Case with the respective adverse event. In other words, the Case Seriousness is cumulative.
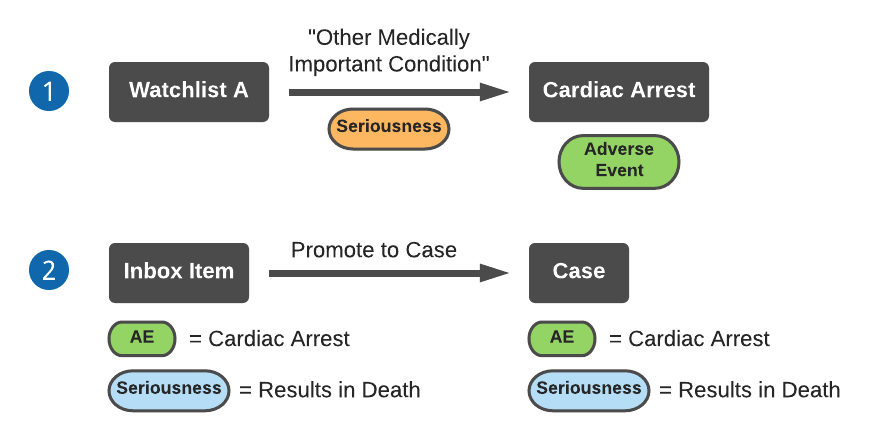
The following diagram describes an example of a watchlist assigning Case Seriousness to an Inbox Item with a specified (not blank) Seriousness field:
- Watchlist A sets the Seriousness to “Other Medically Important Condition” for adverse event Cardiac Arrest.
- After you promote an Inbox Item containing adverse event Cardiac Arrest and a specified Seriousness to a Case, the Inbox Item Seriousness will copy over to the Case. The Watchlist does not update the Case Seriousness.
How to Trigger Watchlist Auto-Calculation
Vault calculates and assigns watchlist tags on a Case following:
- Case promotion.
- Case Adverse Event creation, update, or deletion.
- Case Product creation, update, or deletion.
Vault calculates and assigns DME watchlist tags following:
- Case promotion.
- Case Adverse Event creation, update, or deletion.
- Case Product creation.
- Product or Drug Role update on a Case Product.