Learn how to update your Vault’s configuration to enable Domestic Case Processing.
About the Feature
Support for Domestic Case Processing was added in 21R3. Vaults created in 22R1 or later include the necessary configuration by default, while Vaults originally deployed earlier than 21R3 must have the configuration upgrades described on this page to enable this feature.
Note: Before you enable this feature, the Object Lifecycles and Workflow section of the configuration for Localized Submissions and Translation Support must be completed.
Once enabled, Prepare a Domestic Case describes how to use this feature.
Note: After enabling this feature, enabling Domestic Case Processing for Agency Jurisdictions is recommended.
Update the User Profile Layout
Add the Default Localization field to the User layout:
- In the Admin area, go to Configuration > Pages > User Profile Page.
- Select User Profile Layout.
- Go to the General Information section.
- In the Fields picklist, add the Default Localization before the Language field.
- Save the page.
Configure the Localization Field
- In Business Admin, go to Organizations > Agency (MDFS/NMPA).
- Select the Edit (
 ) icon.
) icon. - In the Localization field, Select:
- Korean (South Korea) for MFDS
OR - Chinese for NMPA
- Korean (South Korea) for MFDS
- Select Save.
Add the Default Localization Field to the User Records Layout
- In Admin, go to Configuration > Objects > User.
- On the Layouts tab, open the User Detail Page Layout.
- Select Add from the General Info section.
- Select Default Localization.
- Select Save.
Update the Case Object
The following configurations are required on the Case object to enable Domestic Case Processing.
Add the Re-generate a Domestic Case Action to the Case Object
- In Admin, go to Configuration > Objects > Case > Actions.
- Select Create.
- Select Re-generate Domestic Case from the dropdown menu.
- Select Continue.
- Select Save.
Add the Re-generate a Domestic Case Action to the Case Object Lifecycle
- In Admin, go to Configuration > Object Lifecycles > Case Lifecycle.
- Select a State. We recommend configuring the action for the Triage state. However, you can configure the action for any state.
- Select Edit from the User Actions section of the state page.
- Select Create Rule.
- Create the Rule form with the following details:
- Always
- Re-generate Domestic Case
- Action Label: Re-generate Domestic Case
- Select Save.
Update the Case Layout
Complete the procedures in this section to update the Case object layout.
Add the Default Localization Field
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open the Case Detail Page Layout.
- Select Add from the System section.
- Select Default Localization.
- Select Save.
(Optional) Create the Hide Default Report Type Layout Rule
- On the Case Page Layout, select Layout Rules.
- Select Create.
- Complete the Create Layout Rule form with the following details:
- Label: Hide Default Report Type
- IF this Layout Expression is TRUE:
""Or(Not(IsBlank(localization__vr.name__v)), (localization__vr.name__v='global__v'))" - Apply the following display effects:
- Effect: Hide
- Type: Field
- Values: Report Type
- Select Save.
(Optional) Remove the Case Narrative Field from the Narrative Section
- Navigate to the Narratives section of the Case Details Page Layout.
- Delete the Case Narrative field.
Replace Standard Case Fields with Control Fields
You must replace the following standard fields with their respective control fields, denoted by a Slider ( ) icon.
) icon.
To replace a standard field with a control field:
Replace the standard fields in the following table with their control field versions:
| Section | Field to Replace With Control Field |
|---|---|
| Patient | Age Group |
| Patient | Medical History Text |
| Narratives | Narrative Preview |
| Narratives | Company Comments |
| Narratives | Reporter's Comments |
| Study | Study Type |
| Study | Study Name (Reason Omitted) |
| Device Details | Device Follow-Up Type |
| Details | Report Type |
| Details | CIOMS Remarks |
Update the Device Follow-up Type Layout Rule
- On the Case Page Layout, select Layout Rules.
- Select Device Follow-up Type.
- Select the Edit (
 ) icon.
) icon. - Change the Type to “Controls”.
- Select Save.
Add the Localization Field to the Child Information Records Layout
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open the Child Information Detail Page Layout.
- Select Add from the System section.
- Select Localization.
- Select Save.
Update the Parental Case Layout
Add the Localization Field to the Child Information Records Layout
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open the Parental Information Detail Page Layout.
- Select Add from the System section.
- Select Localization.
- Select Save.
Replace the Standard Medical History Text Field with the Control Field
- Navigate to the Parent section of the Parental Case Detail Page Layout.
- Remove the Medical History Text standard field.
- Add the Medical History Text control field with the Slider (
 ) icon.
) icon. - Select Done.
Enable Japanese (PMDA) Domestic Case Processing
The following configurations are required to enable Domestic Case Processing for Japan (PMDA).
Update the Case Layout
Complete the procedures in this section to update the Case object layout.
Insert the Case Product Registration Section
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open the Case Page Layout.
- Insert a Related Object section with the following settings:
- Related Object: Case Product Registration
- Section Label: Case Product Registrations
- Creation Option: Create record in new page
- Select Done.
Create the Hide CPR for Non-Japanese Case Layout Rule
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open the Case Page Layout.
- Select Layout Rules.
- Select Create.
- Complete the Create Layout Rule form with the following details:
- Label: Hide CPR for Non-Japanese Case
- IF this Layout Expression is TRUE:
"localization__vr.name__v!='Japanese(Japan)" - Apply the following display effects:
- Effect: Hide
- Type: Sections
- Values: Case Product Registrations
- Select Save.
Update the Case Product Registration Layout
Complete the procedures in this section to update the Case Product Registration object layout.
Update the Details Section
- In Admin, go to Configuration > Objects > Case Product Registration.
- On the Layouts tab, open the Case Product Registration Detail Page Layout.
- Add the following control fields to the Details section:
 OTC Drug Channel
OTC Drug Channel Product
Product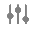 Dose Form
Dose Form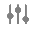 Registration Country
Registration Country
- Select Save.
- Move the Product field to the System section.
- Select Save.
Insert the PMDA Reporting Information Section
- In Admin, go to Configuration > Objects > Case Product Registration.
- On the Layouts tab, open the Case Product Registration Detail Page Layout.
- Insert a Detail Form section with the following details:
- Section Label: PMDA Reporting Information
- Section Layout: Details Form - Two Columns
- Select Done.
- Add the following fields to the PMDA Reporting Information section:
- PMDA Reporting Category
- Completeness
- Immediate Report Type
- Report Overview
- Select Save.
Insert the Case Comments Section
- In Admin, go to Configuration > Objects > Case Product Registration.
- On the Layouts tab, open the Case Product Registration Detail Page Layout.
- Insert a Related Object section with the following details:
- Related Object: Localized Case Comments
- Section Label: Case Comments
- Creation Option: Create record in new page
- Select Save.
Enable China (NMPA) and Korea (MFDS) Domestic Case Processing
The following configurations are required to enable Domestic Case Processing for China (NMPA) and Korea (MFDS).
Update the Case Layout
Complete the procedures in this section to update the Case object layout.
Insert the Regional Section
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open the Case Page Layout.
- Insert a Detail Form section with the following details:
- Section Label: Regional
- Section Layout: Details Form - One Column
- Select Done.
Create the Hide Regional Section for Global and Japanese Cases Layout Rule
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open the Case Page Layout.
- Select Layout Rules.
- Select Create.
- Complete the Create Layout Rule form with the following details:
- Label: Hide Regional Section for Global and Japanese Cases
- IF this Layout Expression is TRUE:
"Or(localization__vr.name__v='Japanese (Japan)', IsBlank(localization__vr.name__v), localization__vr.name__v='Global')" - Apply the following display effects:
- Effect: Hide
- Type: Sections
- Values: Regional
- Select Save.
Update the Case Contact Layout
Complete the procedures in this section to update the Case Contact object layout.
Insert the Regional Section
You must insert the Regional section on the following Case Contact layouts:
- Health Care Professional Detail Page Layout
- Patient Contact Detail Page Layout
- Reporter Detail Page Layout
- Sender Detail Page Layout
- Facility Detail Page Layout
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open a layout.
- Insert a Detail Form section with the following details:
- Section Label: Regional
- Section Layout: Details Form - One Column
- Select Done.
Create the Hide Regional Section for Global and Japanese Cases Layout Rule
You must add the layout rule to the following Case Contact layouts:
- Health Care Professional Detail Page Layout
- Patient Contact Detail Page Layout
- Reporter Detail Page Layout
- Sender Detail Page Layout
- Facility Detail Page Layout
- In Admin, go to Configuration > Objects > Case.
- On the Layouts tab, open a layout.
- Select Layout Rules.
- Select Create.
- Complete the Create Layout Rule form with the following details:
- Label: Hide Regional Section for Global and Japanese Cases
- IF this Layout Expression is TRUE:
"Or(localization__vr.name__v='Japanese (Japan)', IsBlank(localization__vr.name__v), localization__vr.name__v='Global')" - Apply the following display effects:
- Effect: Hide
- Type: Sections
- Values: Regional
- Select Save.
Replace Standard Case Contact Fields with Control Fields
You must replace the following standard fields with their respective control fields, denoted by a Slider (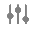 ) icon.
) icon.
To replace a standard field with a control field:
Replace the standard fields in the following table with their control field versions:
| Layout | Section | Field to Replace With Control Field |
|---|---|---|
| Case Contact (All) |
|
Address: Street Line 2 |
| Case Contact (All) |
|
Qualification |
| Case Contact (All) | Sender | Organization |
| Case Contact (All) | Facility |
|
| Case Contact (All) | Sender |
|
Update the Case Product Layout
Complete the procedures in this section to update the Case Product object layout.
Update the Combination Product Layout Rule
You must update the Combination Product layout rule to the following Case Product layouts:
- Biologic Page Layout
- Drug Page Layout
- Device Detail Page Layout
- Vaccine Detail Page Layout
- In Admin, go to Configuration > Objects > Case Product.
- On the Layouts tab, open a layout.
- Select Layout Rules.
- Select Combination Product.
- Add the following to the Apply the following display effects section:
- Effect: Hide
- Type: Controls
- Values: Combination Product
- Select Save.
Update the Blinded Rule Layout Rule
- In Admin, go to Configuration > Objects > Case Product.
- On the Layouts tab, open a Study Product Page Layout.
- Select Layout Rules.
- Select Blinded Rule.
- Add the following to the Apply the following display effects section:
- Effect: Hide
- Type: Controls
- Values: Product (Reported)
- IF this Layout Rule Expression is TRUE:
If(IsBlank(blinded__v), if(IsBlank(case_blinded__v), if(IsBlank(study_blinded__v), FALSE, study_blinded__v), case_blinded__v), blinded__v)
- Select Save.
Replace Standard Case Product Fields with Control Fields
You must replace the following fields with their respective control fields, denoted by a Slider (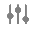 ) icon.
) icon.
To replace a standard field with a control field:
Replace the standard fields in the following table with their control field versions:
| Layout | Section | Field to Replace With Control Field |
|---|---|---|
| Case Product (All) | Details |
|
| Case Product > Device Details Page Layout | Details | Other Additional Information |
| Case Product > Device Details Page Layout | Device Information | Device Usage Type |
| Case Product > Device Details Page Layout | Registration |
|
| Case Product > External Product Page Layout | Other Additional Information | Other Additional Information |
| Case Product > Vaccine Page Layout | Registrations |
|
| Case Product > Study Product Page Layout | Details | Other Additional Information |
| Case Product > Study Product Page Layout | Registrations |
|
Replace Standard Case Child Object Fields with Control Fields
You must replace the following standard fields on the following Case child objects with their respective control fields, denoted by a Slider ( ) icon.
) icon.
To replace a standard field with a control field:
Replace the standard fields in the following table with their control field versions:
| Object | Layout | Section | Field to Replace With Control Field |
|---|---|---|---|
| Case Adverse Event | Case Adverse Event Detail Page Layout | Details |
|
| Case Assessment Result | Case Assessment Result Detail Page Layout | Details |
|
| Case Medical History | Case Medical History Detail Page Layout | Details | Comments |
| Case Test Result | Case Test Result Detail Page Layout | Details |
|
Security
(Optional) Configure Read Permission for the Localization Field
We recommend you set Read permissions on the Localization field of the Case object for all Permission Sets except Vault Owner.
- In Business Admin, go to Users & Groups > Permission Sets > [Permission Set] > Objects > Case > Object Field Permissions > Localization.
- Select Edit.
- Set the Read permission for this field for all roles except Vault Owner.
- Select Save.
Configure Edit Permission for the Session Store ID Field
You must set Edit permissions on the Session Store ID field of the Case object for the following Vault Permission sets:
- Case Distribution
- Case Entry
- Case Intake
- Case Review
- Case Translation
- In Business Admin, go to Users & Groups > Permission Sets > [Permission Set] > Objects > Case > Object Field Permissions > Session Store ID.
- Select Edit.
- Set the Edit permission for this field.
- Select Save.
Add Read and Edit Permissions to All Localized Objects on the Case Entry Permission Set
- In Business Admin, go to Users & Groups > Permission Sets > Case Entry Actions > Objects.
- Select Edit.
- Add Read and Edit permissions to all Localized Case objects:
- Localized Case
- Localized Case Adverse Event
- Localized Case Assessment
- Localized Case Assessment Result
- Localized Case Cause of Death
- Localized Case Comment
- Localized Case Contact
- Localized Case Document
- Localized Case Drug History
- Localized Case Identifier
- Localized Case Medical History
- Localized Case Product
- Localized Case Product Dosage
- Localized Case Product Indication
- Localized Case Product Substance
- Localized Case Test Result
- Localized Controlled Vocabulary
- Localized Country
- Localized Dose Form
- Localized Organization
- Localized Product
- Localized Reason Omitted
- Localized Route of Administration
- Localized Study
- Localized Study Arm
- Localized Substance
- Localized Unit of Measurement
- Select Save.
Add Read Permissions to All Localized Objects on Case Permission Sets
You must Read permissions to the following Case Permission Sets:
- Case Distribution
- Case Intake
- Case Review
- Case Translation
- In Business Admin, go to Users & Groups > Permission Sets > [Permission Set] > Objects.
- Select Edit.
- Add Read permissions to all Localized Case objects:
- Localized Case
- Localized Case Adverse Event
- Localized Case Assessment
- Localized Case Assessment Result
- Localized Case Cause of Death
- Localized Case Comment
- Localized Case Contact
- Localized Case Document
- Localized Case Drug History
- Localized Case Identifier
- Localized Case Medical History
- Localized Case Product
- Localized Case Product Dosage
- Localized Case Product Indication
- Localized Case Product Substance
- Localized Case Test Result
- Localized Controlled Vocabulary
- Localized Country
- Localized Dose Form
- Localized Organization
- Localized Product
- Localized Reason Omitted
- Localized Route of Administration
- Localized Study
- Localized Study Arm
- Localized Substance
- Localized Unit of Measurement
- Select Save.