Learn how to update your Vault’s configuration to enable the Case-level relatedness and SUSAR/SAE tags feature.
About the Feature
Case-level relatedness was added in Veeva Safety 20R1.
This feature introduces the ability to view the Relatedness for the primary assessment at the Case-level.
In addition, new and revised Case Assessments and Cases that meet the appropriate seriousness criteria are automatically tagged as a SUSAR or SAE to aid in ad-hoc reports and data analysis.
Once this feature is enabled, for more information about this feature, refer to the following pages:
- Case Field Reference describes the Relatedness field and the new SUSAR and SAE tags.
- Enter a Case Assessment describes how the Causality Established field on the Assessment Result object rolls up to the Case Relatedness field.
25R3 Update: Tag Only Clinical Trial Study Cases as SUSAR
With the 25R3 release, you can configure your Vault to assign SUSAR tags to Case Assessments only on clinical trial study Cases. If enabled, Vault:
- Applies this logic to new Case Assessment tag assignments.
- Does not update records created before this feature was introduced.
- Reevaluates Assessment Tags on existing Case Assessments if follow-up information is merged into a Case.
If you do not enable this feature, Vault applies the SUSAR tag to any study Case that meets the SUSAR criteria, regardless of the study type. To configure your Vault for the updated feature, see (25R3) Tag Only Clinical Trial Study Cases as SUSAR.
Update the Case Layout
Follow the instructions below to update the Case Page Layout with control sections. Control sections are denoted by the Slider ( ) icon.
) icon.
- Navigate to Admin > Configuration > Objects > Case > Case Page Layout.
- In the Details section, add the Relatedness field.
- If not already present on the layout, insert the Case Assessments control section with the following information:
- Section Label: Assessments
- Section Name: case_assessment_control
- Select Done.
- If not already present on the layout, insert the Case Assessment Results control section with the following information:
- Section Label: Assessment Results
- Section Name: case_assessment_result_control
- Select Done.
- Select Save.
Display Relatedness in Case Hovercard
- Go to Objects > Case > Fields, and then open the Relatedness field.
- Make sure the Display in default lists and hovercards option is enabled.
Update Controlled Vocabularies
- Enable Controlled Vocabulary User Actions to be able to activate and deactivate Controlled Vocabulary records.
- Deactivate any records with the E2B Default Values field set to No or Null, for the following Controlled Vocabularies types:
- Case Assessment Method
- Case Assessment Result
- Case Assessment Source
Note: Deactivating non-E2B default Controlled Vocabularies is optional and may vary between Vaults, depending on each customer’s preferred configuration. The Controlled Vocabularies with the E2B Default Values field set to Yes should not be modified.
Configure Controlled Vocabularies provides more information on managing Controlled Vocabulary records.
Update Triage Workflows
In Veeva Safety 19R3 and earlier, the SUSAR and SAE tags were set through Triage workflows. In 20R1 and later, Vault updates the SUSAR and SAE tags automatically when a Case meets SUSAR or SAE criteria. However, you must update your Vault’s workflow configuration to make this change visible.
Go to Configuration > Object Workflows, and then perform the following steps on the Case Triage and Case Triage (Follow-Up) workflows.
- In both the Case Triage and Case Triage (Follow-Up) workflows, remove the following workflow steps:
- Decision: Is Study?
- Update Record Field: SUSAR
- Update Record Field: SAE
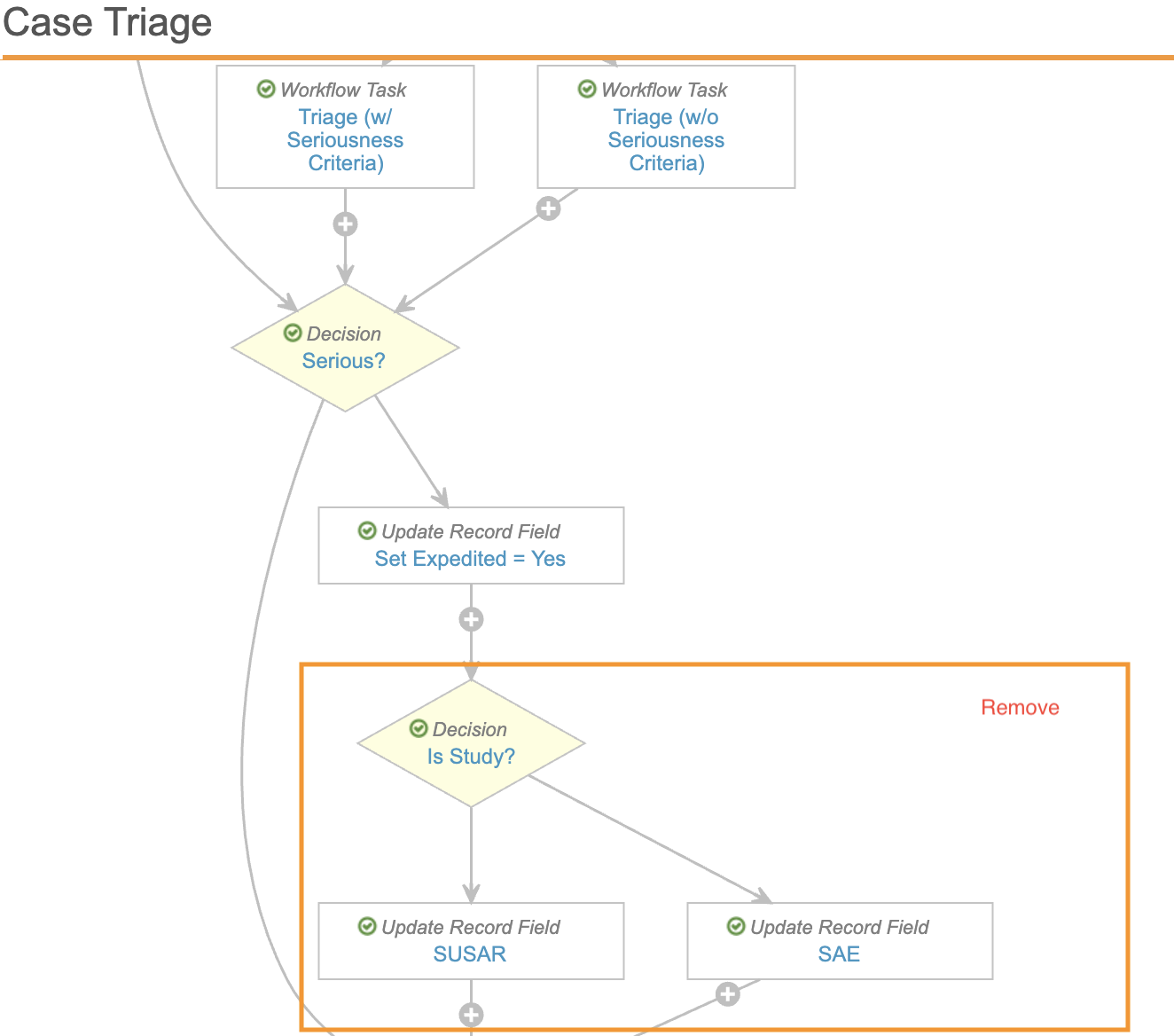
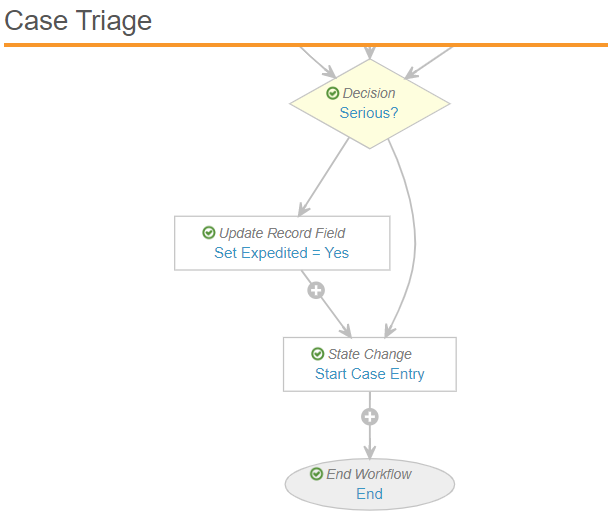
- Link the steps that were before and after the deleted steps to make sure the workflow is valid.
For example, in the Case Triage workflow you may need to update the Set Expedited step to set the next step to Start Case Entry.
(25R3) Tag Only Clinical Trial Study Cases as SUSAR
To configure your Vault to populate SUSAR in the Assessment Tag field on Case Assessments only on clinical trial study Cases:
- Navigate to Admin > Settings > Safety General Settings.
- Select Edit.
- In the Auto-calculation Settings section, select the Only Case Assessments associated with a Clinical Trial study type will be tagged as SUSAR checkbox.
- Select Save.