Learn how to update your Vault’s configuration to enable Case Product section controls for faster data entry.
About the Feature
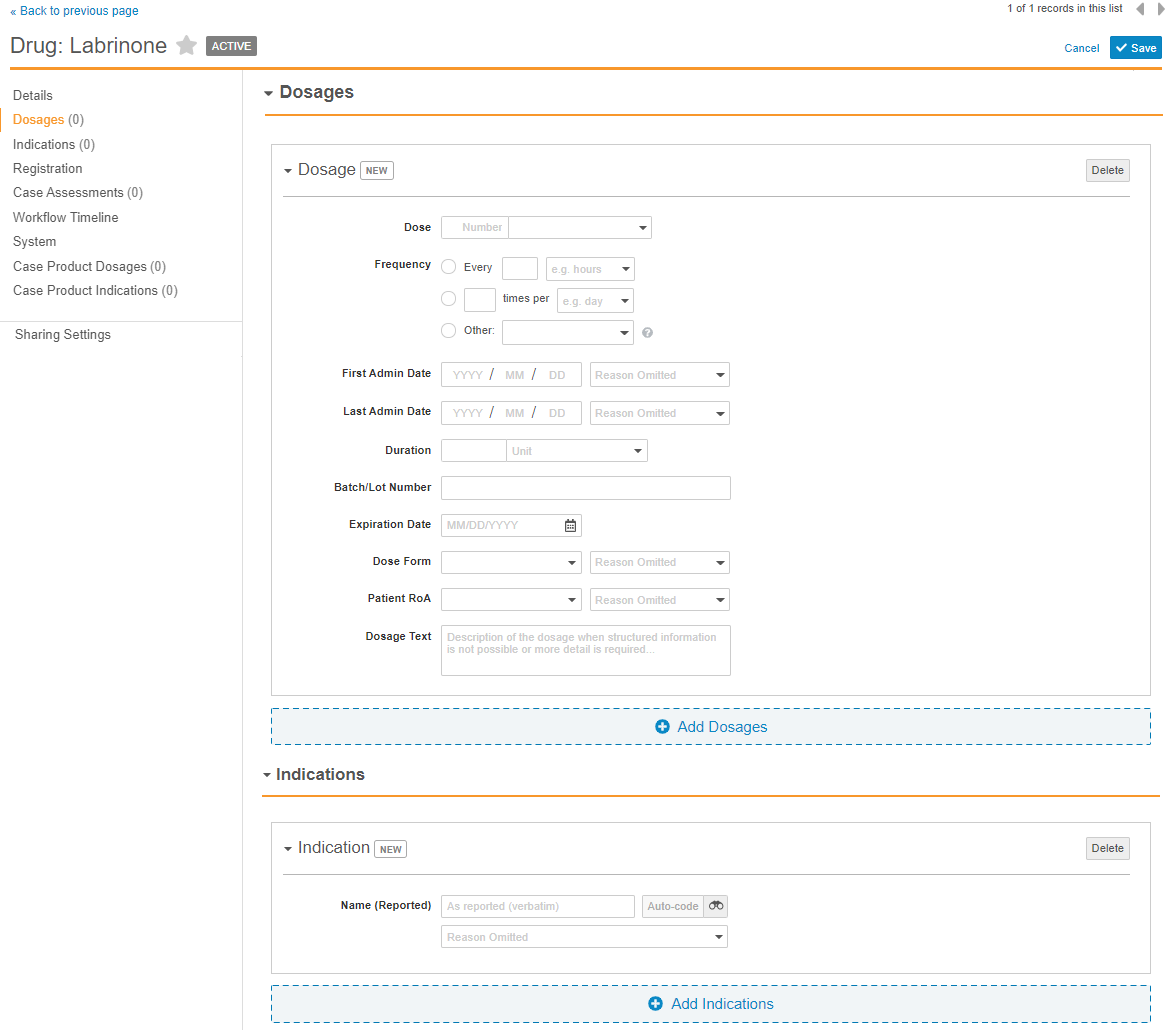
Veeva Safety 20R1 includes new Case Product section controls. This enhancement enables users to enter product dosages and indications directly on the Case Product page, ensuring faster data entry and more intuitive usability.
The following image shows how the Dosages and Indications sections appear when in Edit mode with this feature enabled in your Vault:
For upgraded Vaults, you must perform the following configuration to enable this feature.
Update Case Product Layouts
On the Configuration tab, go to Objects > Case Product > Layouts, and then insert the Dosages and Indications control sections with the Slider ( ) icons on the following layouts:
) icons on the following layouts:
- Biologic Page Layout
- Device Detail Page Layout1
- Drug Page Layout
- External Product Page Layout
- Study Product Page Layout
Note: 1. The Dosages section is not typically required on the Device Detail Page Layout, however this may vary between Vaults.
Replace the Dosages Section
- Remove the Dosages Case Product Dosage related object section from the layout.
- Insert the new Dosages section control.
You can place the section according to preference, however, we recommend that you place this section directly under Details.
Replace the Indications Section
- Remove the Indications Case Product Indication related object section from the layout.
- Insert the new Indications section control.
You can place the section according to preference, however, we recommend that you place this section directly under the new Dosages section.
Update Permission Sets
Follow the steps outlined in Enable xEVMPD Dosage Forms and Non-Standard Dose Units to update your Vault’s permission settings. These same permission set updates are required for users to enter data in the Case Product Section Controls.