Learn how Vault maps Case details to generate the European Commission’s Manufacturer Incident Report (MIR) for Serious Incidents (MDR/IVDR) and Incidents (AIMDD/MDD/IVDD), Version 7.3.1. Depending on your Transmission Document Type selection during Transmission generation, Vault formats the document as a PDF (EU MIR) or an XML file (EU MIR XML). When generating XML files, Vault truncates values to fit within data element character limits.
In the sections below, images of the form represent the PDF format, but the data mapping descriptions in the tables apply to both the PDF and the XML formats.
Prerequisite
Your Admin must enable European Union Manufacturer Incident Report (EU MIR) PDF Generation.
Section 1 captures information about the responsible competent authority, details about the incident report, information on the report submitter, device manufacturer, and authorized representative.
1.1 Responsible competent authority in which country the incident occurred
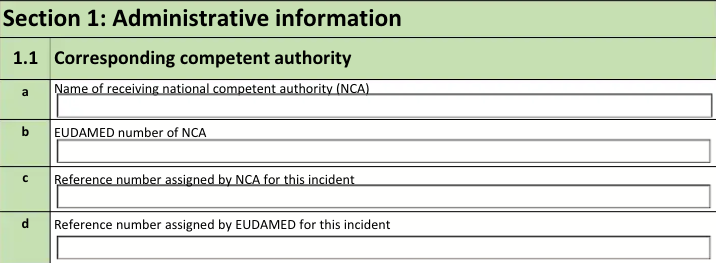
The form fields include:
| MIR Form Field |
Data Source |
| a. Name of receiving national competent authority (NCA) |
The Name of the Agency in the Destination field of the Transmission. |
| b. EUDAMED number of NCA |
The EUDAMED Number of the Agency in the Destination field of the Transmission. |
| c. Reference number assigned by NCA for this incident |
The NCA Reference Number on the Device Incident Report of the Device-type Case Product. If blank, Vault populates Not Known. |
| d. Reference number assigned by EUDAMED for this incident |
The EUDAMED Reference Number on the Device Incident Report of the Device-type Case Product. If blank, Vault populates Not Known. |
1.2 Date, type, and classification of incident report
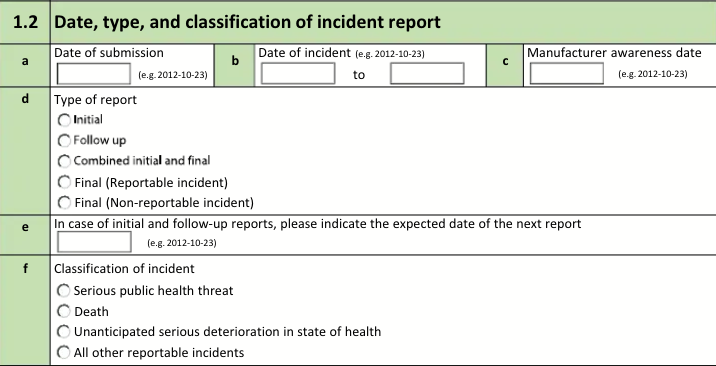
The form fields include:
| MIR Form Field |
Data Source |
| a. Date of report submission |
The Transmission Date on the Transmission. |
| b. Date of incident |
Vault populates a date range for when the onset of the event could have occurred based on the Onset date of the primary Case Adverse Event:
- If the Onset date includes the day, month, and year, Vault populates from
yyyy-mm-dd to yyyy-mm-dd.
- If the Onset date includes only the month and year, Vault populates from the first to the last day of the month. For example, if the Onset date is April 2026, Vault populates from
2026-04-01 to 2026-04-30.
- If the Onset date includes only the year, Vault populates from the first to the last day of the year. For example, if the Onset date is 2026, Vault populates from
2026-01-01 to 2026-12-31.
Note: If the Transmission Date is before the last date of the range, Vault exports the Transmission Date in the second field. For example, if the Onset date is 2025 and the Transmission Date is September 23, 2025, Vault populates from 2025-01-01 to 2025-09-23.
If the Onset date is blank, Vault populates the Transmission Date.
|
| c. Manufacturer awareness date of the incident |
The Initial Receipt Date on the Case. |
| d. Manufacturer awareness date of reportability |
The Manufacturer Reportable Awareness Date on the Case. |
| e. Type of report |
The Type of Incident Report on the Device Incident Report of the Device-type Case Product. |
| f. In case of initial and follow-up reports, please indicate the expected date of the next report |
The Expected Date of Next Report on the Device Incident Report of the Device-type Case Product. |
| g. Classification of serious incident |
Vault selects an option using the following priority:
- Death: If the Seriousness of the Case Adverse Event includes Results in Death
- Serious public health threat: If the Device Report Type of the Device-type Case Product includes Public Health Risk and the Case Adverse Event includes a Seriousness value
- Unanticipated serious deterioration in state of health: If the Device Report Type of the Device-type Case Product does not include Public Health Risk and the Case Adverse Event includes a Seriousness value and the Case Assessment Expectedness has No in the Expected field
- All other reportable incidents: In all other scenarios
|
This includes information about the report submitter, manufacturer, and additional sections that Vault may populate depending on the type of report submitter.
1.3.1 Submitter of the report
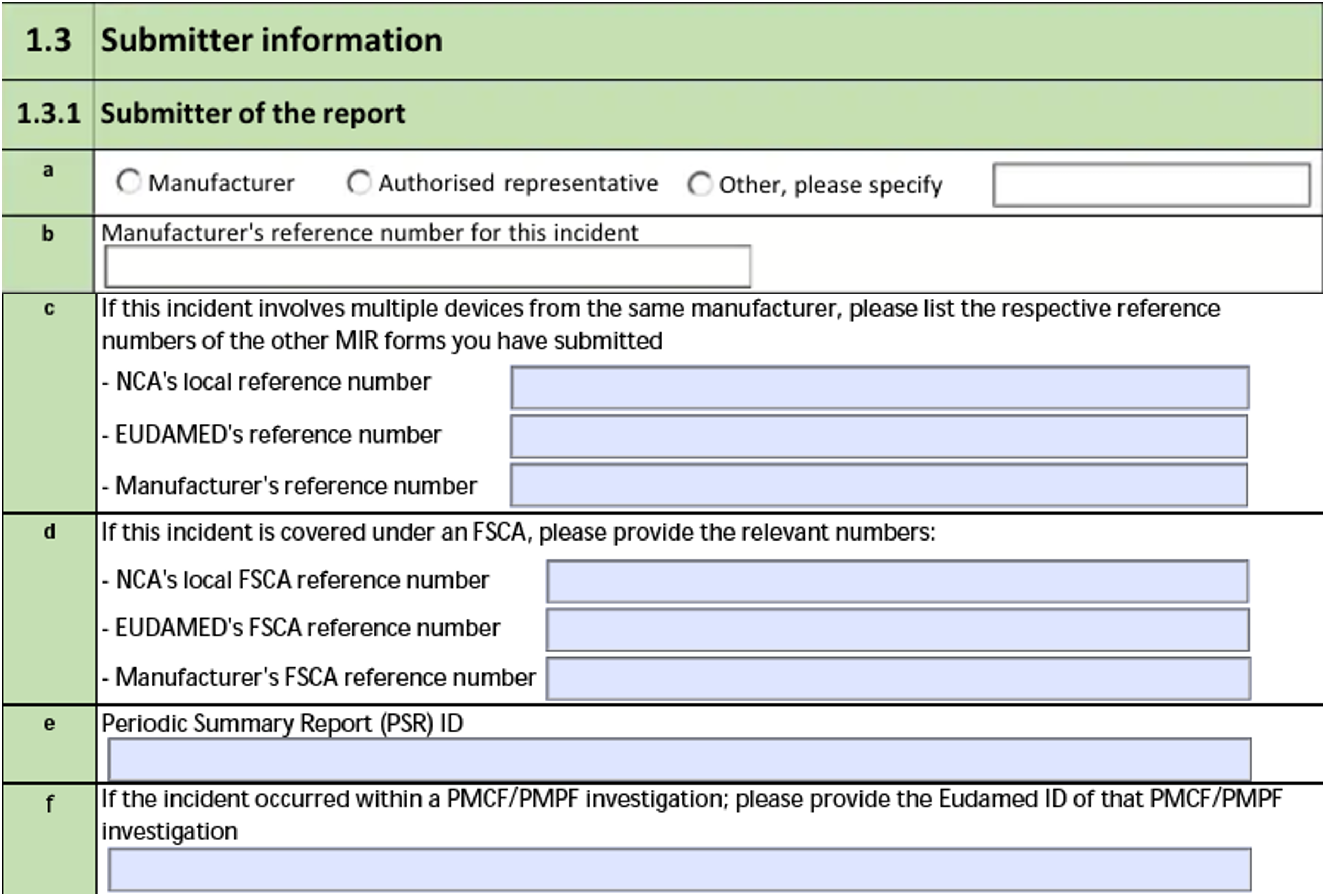
The form fields include:
| MIR Form Field |
Data Source |
| a. Submitter of the report |
The Submitter of Report on the Device Incident Report of the Device-type Case Product.
When the Submitter of Report is Other, Vault also exports the Submitter of Report (Other) text. |
| b. Manufacturer's reference number for this incident |
The Manufacturer Reference Number on the Device Incident Report of the Device-type Case Product. |
| c. NCA's local reference number |
If any other Case Products include an NCA Reference Number, Vault exports the value. Vault comma-separates multiple values. |
| c. EUDAMED's reference number |
If any other Case Products include a EUDAMED Number, Vault exports the value. Vault comma-separates multiple values. |
| c. Manufacturer's reference number |
If any other Case Products include a Manufacturer Reference Number, Vault exports the value. Vault comma-separates multiple values. |
| d. NCA's local FSCA reference number |
The NCA FSCA Reference Number on the Device Incident Report of the Device-type Case Product. |
| d. EUDAMED's FSCA reference number |
The EUDAMED FSCA Reference Number on the Device Incident Report of the Device-type Case Product. |
| d. Manufacturer's FSCA reference number |
The Manufacturer FSCA Reference Number on the Device Incident Report of the Device-type Case Product. |
| e. Periodic Summary Report (PSR) ID |
The Periodic Summary Report (PSR) ID from the Case Product. |
| f. The incident occurred within a PMCF/PMPF investigation |
For study Cases with a Study Type of Other and a Registration Type that corresponds to E2B code 7, Vault selects yes. Otherwise, Vault selects no. |
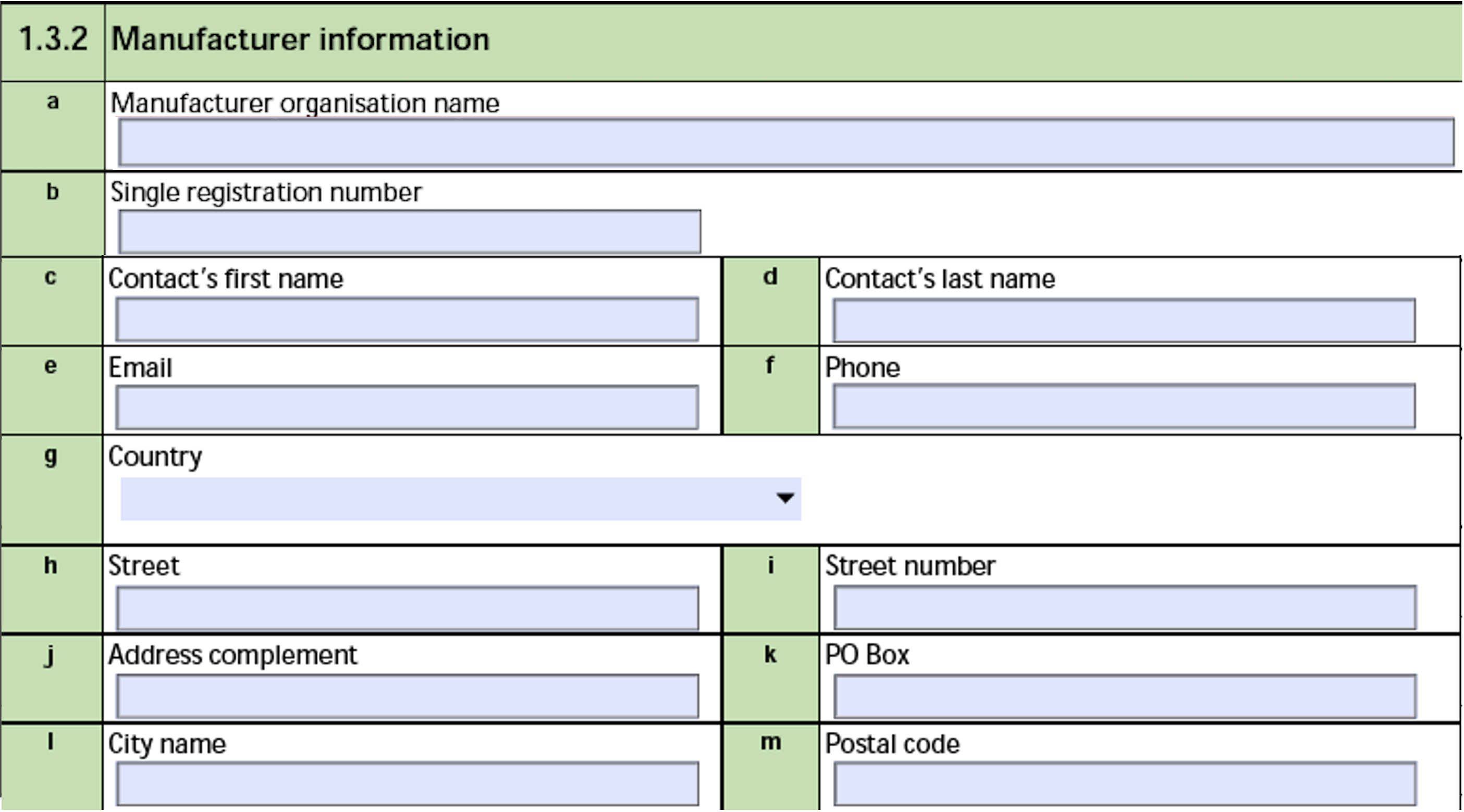
The form fields include:
| MIR Form Field |
Data Source |
| a. Manufacturer organisation name |
The Name of the Organization linked in the Manufacturer field of the corresponding Product record. |
| b. Single registration number |
The Single Registration Number (SRN) of the Organization linked in the Manufacturer field of the corresponding Product record. |
| c. Contact's first name |
The First Name of the User associated with the Sender (User) on the Transmission. |
| d. Contact's last name |
The Last Name of the User associated with the Sender (User) on the Transmission. |
| e. Email |
The Email of the User associated with the Sender (User) on the Transmission. |
| f. Phone |
The value in the Telephone field of the User associated with the Sender (User) on the Transmission. |
| g. Country |
The two-letter ISO code associated with the Country of the User associated with the Sender (User) on the Transmission. |
| h. Street |
The value, excluding leading numbers, in the Street field of the User associated with the Sender (User) on the Transmission. Vault does not populate this field if the street address includes a PO Box. |
| i. Street number |
The leading numbers in the Street field of the User associated with the Sender (User) on the Transmission. |
| j. Address complement |
Vault does not populate this field. |
| k. PO Box |
If the Street field of the User associated with the Sender (User) on the Transmission includes a PO Box, Vault populates the Street value. |
| l. City name |
The City of the User associated with the Sender (User) on the Transmission. |
| m. Postal code |
The Postal Code of the User associated with the Sender (User) on the Transmission. |
Vault populates this section only if the Submitter of Report is Authorised Representative.
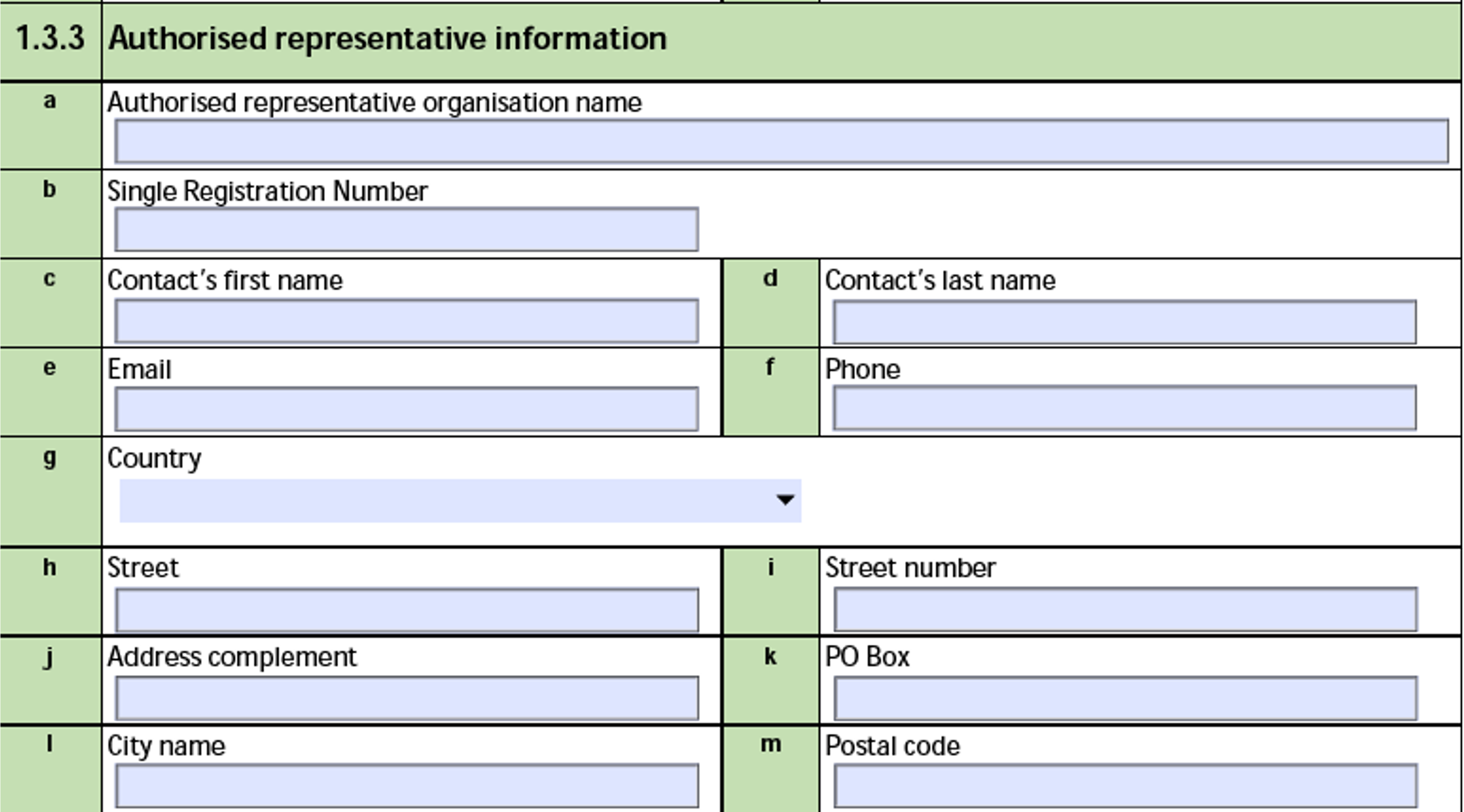
The form fields include:
| MIR Form Field |
Data Source |
| a. Authorised representative organisation name |
The Name of the Organization associated with the Sender (User) on the Transmission. |
| b. Single Registration Number |
The Single Registration Number (SRN) of the Organization associated with the Sender (User) on the Transmission. |
| c. Contact's first name |
The First Name of the User associated with the Sender (User) on the Transmission. |
| d. Contact's last name |
The Last Name of the User associated with the Sender (User) on the Transmission. |
| e. Email |
The Email of the User associated with the Sender (User) on the Transmission. |
| f. Phone |
The value in the Telephone field of the User associated with the Sender (User) on the Transmission. |
| g. Country |
The two-letter ISO code associated with the Country of the User associated with the Sender (User) on the Transmission. |
| h. Street |
The value, excluding leading numbers, in the Street field of the User associated with the Sender (User) on the Transmission. Vault does not populate this field if the street address includes a PO Box. |
| i. Street number |
The leading numbers in the Street field of the User associated with the Sender (User) on the Transmission. |
| j. Address complement |
Vault does not populate this field. |
| k. PO Box |
If the Street field of the User associated with the Sender (User) on the Transmission includes a PO Box, Vault populates the Street value. |
| l. City name |
The City of the User associated with the Sender (User) on the Transmission. |
| m. Postal code |
The Postal Code of the User associated with the Sender (User) on the Transmission. |
1.3.4 Submitter’s details if not also manufacturer or authorised representative
Vault populates this section only if the Submitter of Report is Other.
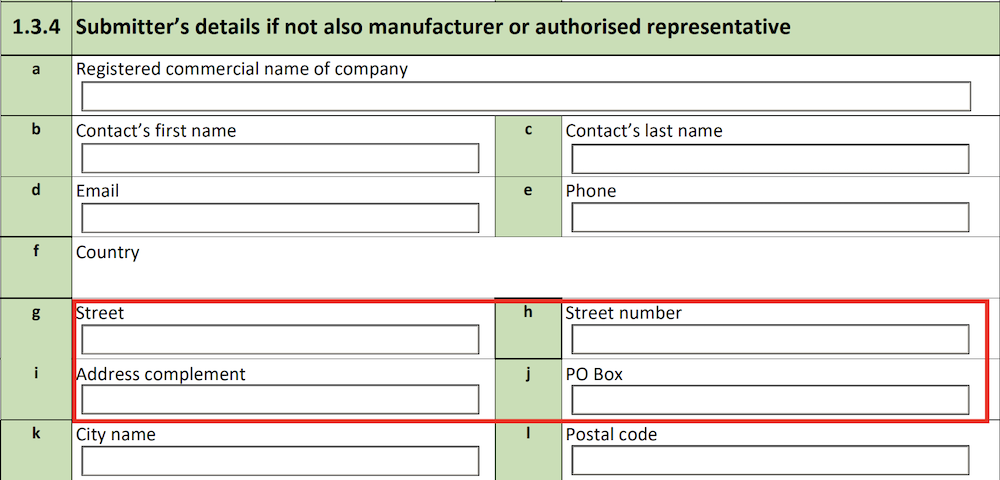
The form fields include:
| MIR Form Field |
Data Source |
| a. Registered commercial name of company |
The Name of the Organization associated with the Sender (User) on the Transmission. |
| b. Contact's first name |
The First Name of the User associated with the Sender (User) on the Transmission. |
| c. Contact's last name |
The Last Name of the User associated with the Sender (User) on the Transmission. |
| d. Email |
The Email of the User associated with the Sender (User) on the Transmission. |
| e. Phone |
The value in the Telephone field of the User associated with the Sender (User) on the Transmission. |
| f. Country |
The two-letter ISO code associated with the Country of the User associated with the Sender (User) on the Transmission. |
| g. Street |
The value, excluding leading numbers, in the Street field of the User associated with the Sender (User) on the Transmission. Vault does not populate this field if the street address includes a PO Box. |
| h. Street number |
The leading numbers in the Street field of the User associated with the Sender (User) on the Transmission. |
| i. Address complement |
Vault does not populate this field. |
| j. PO Box |
If the Street field of the User associated with the Sender (User) on the Transmission includes a PO Box, Vault populates the Street value. |
| k. City name |
The City of the User associated with the Sender (User) on the Transmission. |
| l. Postal code |
The Postal Code of the User associated with the Sender (User) on the Transmission. |
Section 2 includes device identification, categorization, description, and risk class information, as well as identifies the market distribution of the device and the use of relevant related accessories and devices.
2.1 Unique Device Identification (UDI)

The form fields include:
| MIR Form Field |
Data Source |
| a. (Master) UDI-DI/Eudamed ID |
The Unique Identifier on the Device Information record of the Device-type Case Product. |
| a. Issuing entity |
The Unique Identifier Issuing Entity on the Device Information record of the Device-type Case Product. |
| b. UDI production identifier |
The UDI Production Identifier on the Device Information record of the Device-type Case Product. |
| c. Basic UDI-DI/Eudamed-DI |
Vault populates a value using the following priority order:
- The Basic UDI-DI on the Device-type Case Product.
- The Basic UDI-DI on the Product related to the Device-type Case Product.
|
| c. Issuing entity |
The Basic UDI-DI Issuing Entity on the Device Information record of the Device-type Case Product. |
| d. Unit of use UDI-DI |
The Unit of Use UDI-DI on the Device Information record of the Device-type Case Product. |
| d. Issuing entity |
The Unit of Use UDI-DI Issuing Entity on the Device Information record of the Device-type Case Product. |
2.2 Categorisation of device
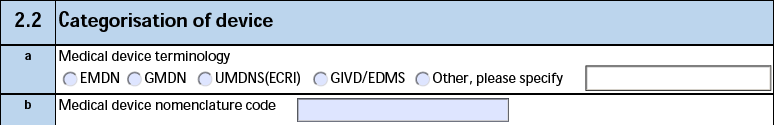
The form fields include:
| MIR Form Field |
Data Source |
| a. Medical device nomenclature |
The Medical Device Terminology on the Device Incident Report of the Device-type Case Product. |
| a. if other, please specify |
When the Medical Device Terminology is Other, Vault also exports the Medical Device Terminology (Other) text. |
| b. Medical device nomenclature code |
Vault populates a value using the following priority:
- The Device Nomenclature Code on the Product related to the Case Product.
- The Device Nomenclature Code on the Device Information record of the Device-type Case Product.
|
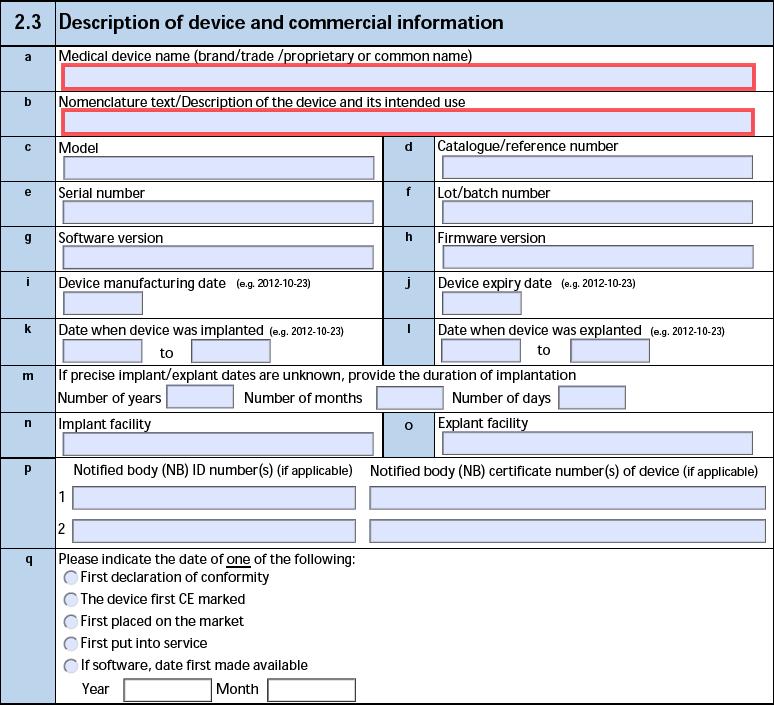
The form fields include:
| MIR Form Field |
Data Source |
| a. Medical device name (brand/trade /proprietary or common name) |
The Brand Name on the Product related to the Device-type Case Product. |
| b. Description of the device and its intended purpose |
The Description of Device on the Product related to the Device-type Case Product. |
| b. Nomenclature text |
The Nomenclature Text on the Product related to the Device-type Case Product. |
| c. Model |
The Model Number on the Device Information record of the Device-type Case Product. |
| d. Catalogue/reference number |
The Catalog Number on the Device Information record of the Device-type Case Product. |
| e. Serial number |
The Serial Number on the Device Information record of the Device-type Case Product. |
| f. Lot/batch number |
Vault populates a value using the following priority:
- The Batch/Lot Number of the Case Product Dosage
- The Lot Number in the Device Information section of the Device-type Case Product
|
| g. Software version |
The Software Version on the Device Information record of the Device-type Case Product. |
| h. Firmware version |
The Firmware Version on the Device Information record of the Device-type Case Product. |
| i. Device manufacturing date |
The Manufacture Date on the Device Information record of the Device-type Case Product. |
| j. Device expiry date |
The Expiration Date on the Device-type Case Product. If blank, Vault exports the earliest Expiration Date on any Case Product Dosage record on the Device-type Case Product. |
| k. Date when device was implanted |
The Date Implanted on the Device Information record of the Device-type Case Product. If only a partial date exists, Vault populates 01 for the missing month and day as needed. For example, if the Onset date includes only the year 2025, Vault populates 2025-01-01. |
| l. Date when device was explanted |
The Date Explanted on the Device Information record of the Device-type Case Product. If only a partial date exists, Vault populates 01 for the missing month and day as needed. For example, if the Onset date includes only the year 2025, Vault populates 2025-01-01. |
| m. If precise implant/explant dates are unknown, provide the duration of implantation |
If both the Date Implanted and Date Explanted are available and include exact dates, Vault populates the date range of implantation. Vault does not export partial dates. |
| n. Implant facility |
The Implant Facility on the Device Information record of the Device-type Case Product. |
| o. Explant facility |
The Explant Facility on the Device Information record of the Device-type Case Product. |
| p. Notified body (NB) ID number(s) Notified body (NB) certificate number(s) of device |
The Notified Body ID and Notified Body Certificate Number on the Product Registration of the Device-type Case Product. |
| q. Please indicate the date of one of the following |
The Device Market Date Type and Device Market Date on the Product Registration of the Device-type Case Product. |
2.4 Risk class of device when placed on market
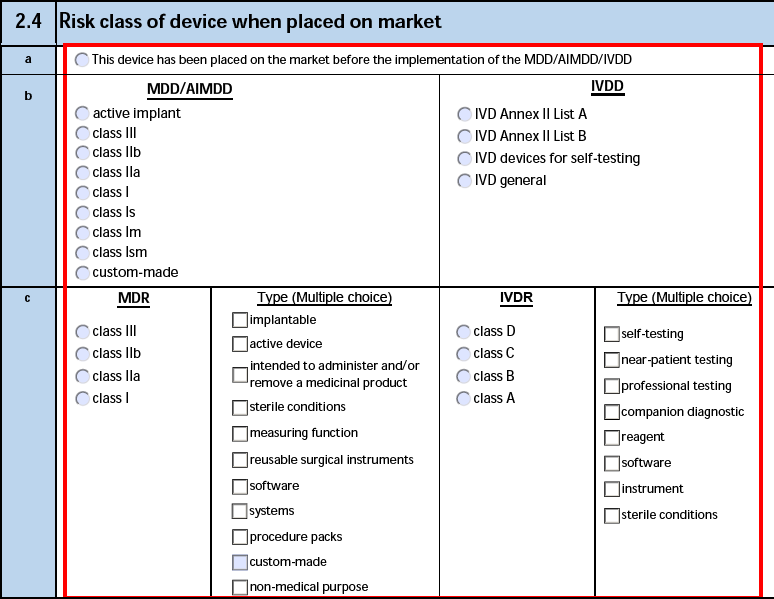
The form fields include:
| MIR Form Field |
Data Source |
| a. Applicable legislation unknown |
If the Applicable Legislation Unknown value is Yes on the Product Registration, Vault selects this option. |
| b. This device has been placed on the market before the implementation of the MDD/AIMDD/IVDD |
Vault selects the option if the Product Registration of the Device-type Case Product meets both criteria:
- The MDD/AIMDD Risk Class is Marketed Before MDD/AIMDD.
- The IVDD Risk Class is Marketed Before IVDD.
|
| c. MDD/AIMDD |
The MDD/AIMDD Risk Class on a Product Registration using the following priority:
- The MDD/AIMDD Risk Class of a Product Registration linked to the Case Product.
- The MDD/AIMDD Risk Class of a Product Registration in a country where the Case Adverse Event occurred. Vault exports the first found record with a value.
- The MDD/AIMDD Risk Class of a Product Registration in a country under the jurisdiction of the EMA. Vault exports the first found record with a value.
Vault does not populate this field when the MDD/AIMDD Risk Class is Marketed Before MDD/AIMDD. |
| c. IVDD |
The IVDD Risk Class on a Product Registration using the following priority:
- The IVDD Risk Class of a Product Registration linked to the Case Product.
- The IVDD Risk Class of a Product Registration in a country where the Case Adverse Event occurred. Vault exports the first found record with a value.
- The IVDD Risk Class of a Product Registration in a country under the jurisdiction of the EMA. Vault exports the first found record with a value.
Vault does not populate this field when the IVDD Risk Class is Marketed Before IVDD. |
| d. MDR |
The MDR Class on a Product Registration using the following priority:
- The MDR Class of a Product Registration linked to the Case Product.
- The MDR Class of a Product Registration in a country where the Case Adverse Event occurred. Vault exports the first found record with a value.
- The MDR Class of a Product Registration in a country under the jurisdiction of the EMA. Vault exports the first found record with a value.
|
| d. Type |
The MDR Type on a Product Registration using the following priority:
- The MDR Type of a Product Registration linked to the Case Product.
- The MDR Type of a Product Registration in a country where the Case Adverse Event occurred. Vault exports the first found record with a value.
- The MDR Type of a Product Registration in a country under the jurisdiction of the EMA. Vault exports the first found record with a value.
|
| d. IVDR |
The IVDR Class on a Product Registration using the following priority:
- The IVDR Class of a Product Registration linked to the Case Product.
- The IVDR Class of a Product Registration in a country where the Case Adverse Event occurred. Vault exports the first found record with a value.
- The IVDR Class of a Product Registration in a country under the jurisdiction of the EMA. Vault exports the first found record with a value.
|
| d. Type |
The IVDR Type on a Product Registration using the following priority:
- The IVDR Type of a Product Registration linked to the Case Product.
- The IVDR Type of a Product Registration in a country where the Case Adverse Event occurred. Vault exports the first found record with a value.
- The IVDR Type of a Product Registration in a country under the jurisdiction of the EMA. Vault exports the first found record with a value.
|
| e. Did this device continue to be placed on the EU market after MDR / IVDR date of application? |
The EU Market after application date on the Device Information record of the Device-type Case Product. If blank, Vault exports the EU Market After Application Date from the Product Registration. |
| f. Does the device fulfil any of the following cases: |
If either of the Scientific Opinion Asked or Competent Authority Consulted fields on the Device Information record of the Device-type Case Product is Yes, Vault selects Yes. Otherwise Vault selects No. |
| f. Competent authority name or European Medicines Agency which delivered the scientific opinion or was consulted by the notified body |
The Competent Authority Consulted Name on the Device Information record of the Device-type Case Product. |
| f. Name(s) of the medicinal substance(s) / product(s), tissue(s), cell(s) of human origin or their derivative(s) associated with the device |
The Associated Products on the Device Information record of the Device-type Case Product. |
2.5 Market distribution of device (region/country)
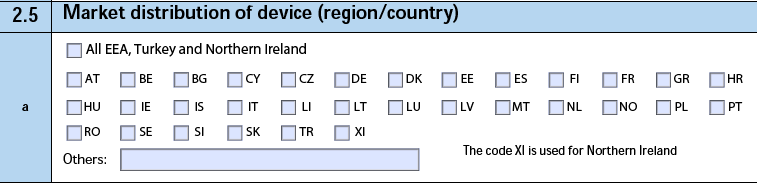
The form fields include:
| MIR Form Field |
Data Source |
| a. Market distribution of device (region/country) |
Vault selects each country code where the Case Product has a Product Registration. If the Case Product is registered in all countries, Vault selects the All EEA, Switzerland and Turkey checkbox. If the Case Product is registered in additional countries, Vault populates the country codes in the Others field, separating values with a semicolon (;). |
2.6 Use of accessories, associated devices or other devices
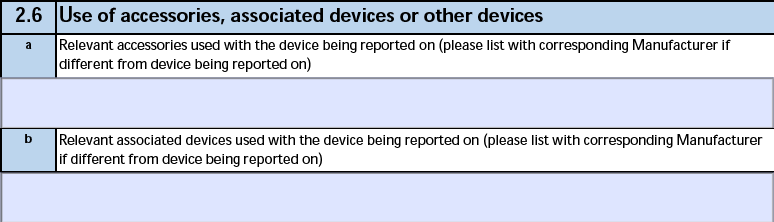
The form fields include:
| MIR Form Field |
Data Source |
| a. Relevant accessories used with the device being reported on |
The Relevant Accessories on the Device Information record of the Device-type Case Product. |
| b. Relevant associated devices used with the device being reported on |
The Relevant Associated Devices on the Device Information record of the Device-type Case Product. |
Section 3 includes details about the nature of the incident, medical device problem, patient, and initial reporter.
3.1 Nature of incident
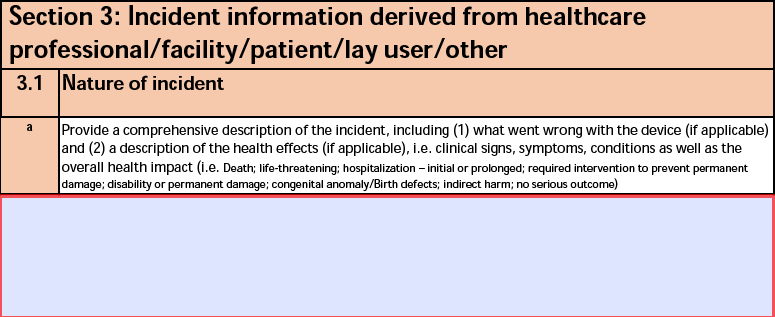
The form fields include:
| MIR Form Field |
Data Source |
| a. Provide a comprehensive description of the incident |
The Additional Device Manufacturer Narrative on the Case Product. |
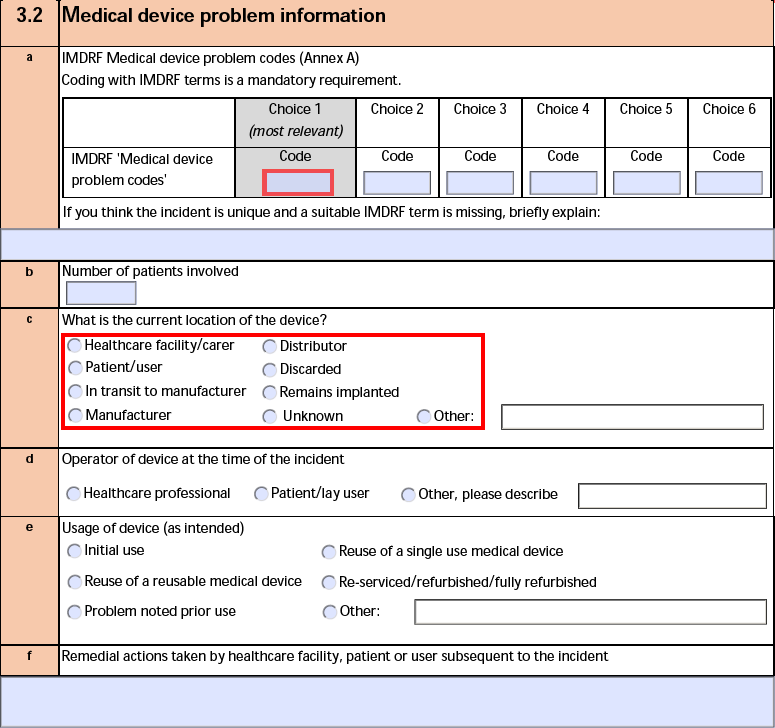
The form fields include:
| MIR Form Field |
Data Source |
| a. IMDRF Medical device problem codes (Annex A) |
If the Device Type Code of the Device-type Case Product is Medical Device Problem, Vault exports an IMDRF code using the following priority:
- Level 3
- Level 2
- Level 1
|
| b. Number of patients involved |
The Number of Patients Involved from the Case Product. If blank, Vault populates 1. |
| c. What is the current location of the device? |
The Current Device Location on the Device Information record of the Device-type Case Product.
When the Current Device Location is Other, Vault also exports the Current Device Location (Other) text. |
| d. Operator of device at the time of the incident |
The Operator of Device on the Device Information record of the Device-type Case Product.
When the Operator of Device is Other, Vault also exports the Operator of Device (Other) text. |
| e. Usage of device (as intended) |
The Device Usage Type value and the Usage of Device (Other) text on the Device Information record of the Device-type Case Product. |
| f. Remedial actions taken by healthcare facility, patient or user subsequent to the incident |
The Remedial Action and Remedial Action (Other) values from the Case Product. Vault comma-separates multiple values. |
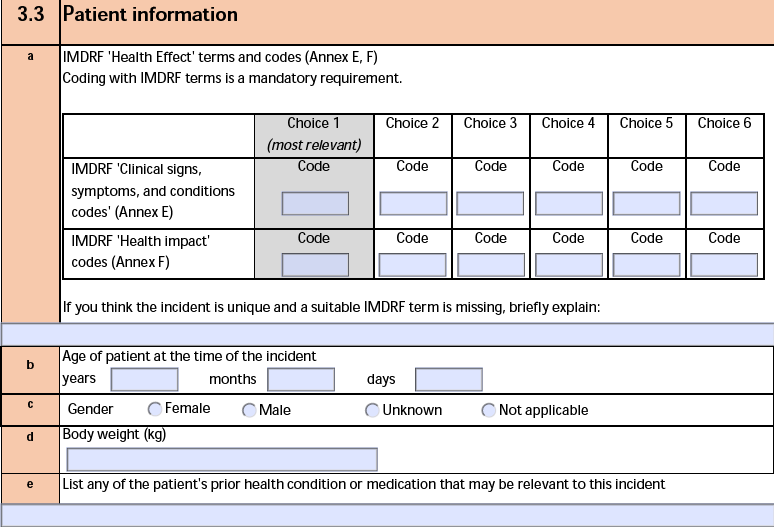
The form fields include:
| MIR Form Field |
Data Source |
| a. IMDRF 'Health Effect' terms and codes (Annex E, F) |
If the Device Type Code of the Device-type Case Product is Health Effects - Clinical signs and Symptoms or Conditions or Health Effects - Health Impact, Vault exports an IMDRF code based on the Rank field of the Case Product Device Code, if populated. Otherwise, Vault exports a code using the following priority:
- Level 3
- Level 2
- Level 1
|
| b. Age of patient at the time of the incident |
The Age (normalized) of the Case Patient. If the age value is two (2) years or less, Vault exports the age in years, months, and days. Otherwise, Vault populates only the year, rounding down to the nearest year. |
| c. Gender |
The value in the Sex field on the Case as follows:
- If Female, Vault selects Female.
- If Male, Vault selects Male.
- In either of the following instances, Vault selects Other:
- Sex is blank, but Gender includes a value.
- Both Gender and Gender (reason omitted) include values.
- If blank and Gender (reason omitted) is blank, Not Asked, Unknown, or Asked But Unknown, Vault selects Not specified.
|
| d. Body weight |
The Weight (normalized) of the Case Patient. |
| e. Height (cm) |
The Height (normalized) of the Case Patient. |
| f. List any of the patient's prior health condition or medication that may be relevant to this incident |
The Device Usage Type value and the Usage of Device (Other) text on the Device Information record of the Device-type Case Product. |
3.4 Initial reporter (can be healthcare professional of facility, patient, lay user)
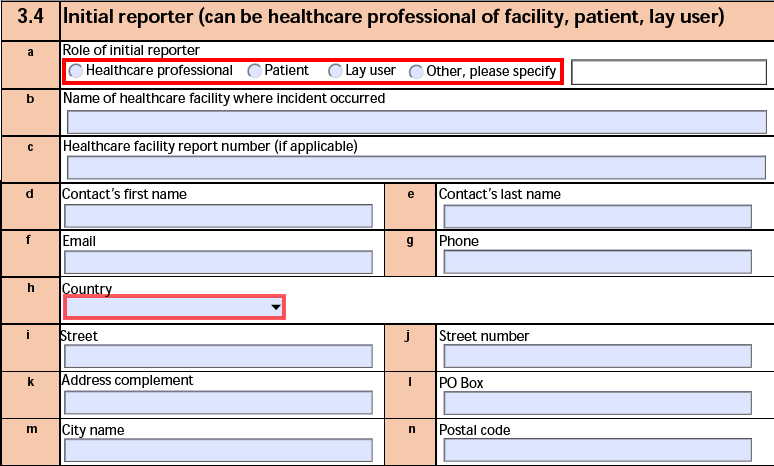
The form fields include:
| MIR Form Field |
Data Source |
| a. Role of initial reporter |
The Qualification of the primary Reporter-type Case Contact. |
| b. Name of healthcare facility where incident occurred |
The Facility Name or Facility Name (Reason Omitted) of the primary Reporter-type Case Contact. |
| c. Healthcare facility report number |
Vault does not populate this field. |
| d. Contact's first name |
The First Name of the primary Reporter-type Case Contact. |
| e. Contact's last name |
The Last Name of the primary Reporter-type Case Contact. |
| f. Email |
The Email Address of the primary Reporter-type Case Contact. |
| g. Phone |
The Telephone value of the primary Reporter-type Case Contact. |
| h. Country |
The Country of the primary Reporter-type Case Contact. If the country is not in the drop-down list, Vault:
- Populates Other in the Country field.
- Selects the if other, please specify checkbox.
- Exports the name of the Country in the text box.
|
| i. Street |
The Street of the primary Reporter-type Case Contact. |
| j. Street number |
The Street Number of the primary Reporter-type Case Contact. |
| k. Address complement |
The Street Line 2 value of the primary Reporter-type Case Contact. |
| l. PO Box |
The PO Box of the primary Reporter-type Case Contact. |
| m. City name |
The City of the primary Reporter-type Case Contact. |
| n. Postal code |
The Postal Code of the primary Reporter-type Case Contact. |
Section 4: Manufacturer analysis
Section 4 includes the manufacturer’s preliminary comments, cause investigation and conclusion details, and similar incident information.
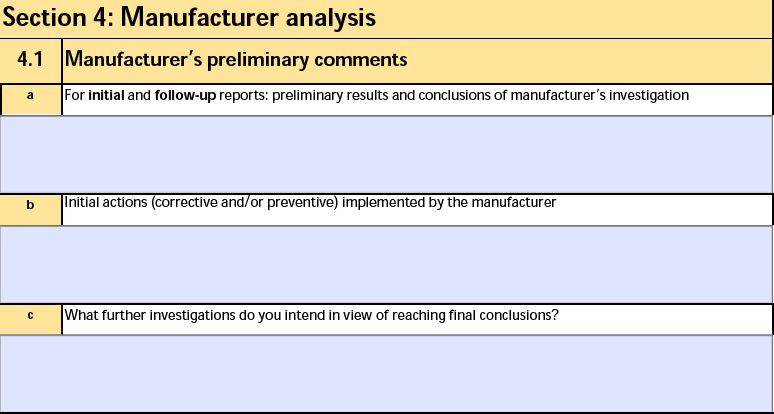
The form fields include:
| MIR Form Field |
Data Source |
| a. For initial and follow-up reports: preliminary results and conclusions of manufacturer's investigation |
The Preliminary Results and Conclusions text on the Manufacturer Analysis of the Device-type Case Product. |
| b. Suspicion of a relationship between the incident and the medicinal substance(s) / product(s), tissue(s), cell(s) of human origin or their derivative(s) associated with the device? |
The Initial Product and Incident Assessment on the Device Information record of the Device-type Case Product. |
| c. Initial actions (corrective and/or preventive) implemented by the manufacturer |
The Initial (Corrective/Preventative) Actions text on the Manufacturer Analysis of the Device-type Case Product. |
| d. What further investigations do you intend in view of reaching final conclusions? |
The Further Investigations text on the Manufacturer Analysis of the Device-type Case Product. |
4.2 Cause investigation and conclusion
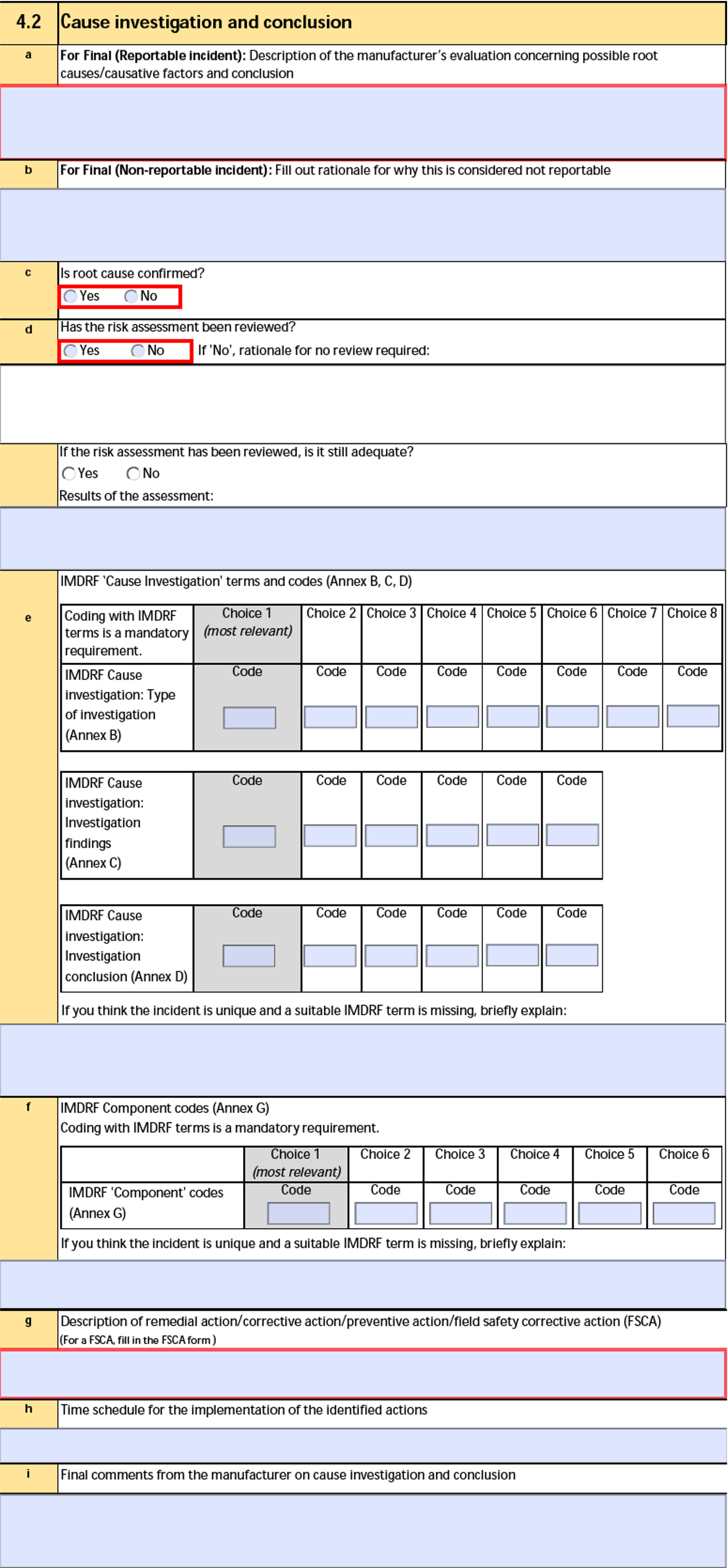
The form fields include:
| MIR Form Field |
Data Source |
| a. For Final (Serious incident): Description of the manufacturer's evaluation concerning possible root causes/causative factors and conclusion |
The Root Cause Description and Conclusion text on the Manufacturer Analysis of the Device-type Case Product. |
| b. For Final (Non-reportable incident): Fill out rationale for why this is considered not reportable |
The Not Reportable Rationale on the Device Incident Report of the Device-type Case Product. |
| c. Is root cause confirmed? |
The Is root cause confirmed? value on the Manufacturer Analysis of the Device-type Case Product. |
| c. Suspicion or confirmation of a relationship between the serious incident and the medicinal substance(s)/product(s), tissue(s), cell(s) of human origin or their derivative(s) associated with the device |
The Final Product and Incident Assessment on the Device Information record of the Device-type Case Product. |
| d. Has the risk assessment been reviewed? |
The Risk assessment reviewed? value on the Manufacturer Analysis of the Device-type Case Product. If the risk assessment has not been reviewed, Vault populates the Rationale for no review required value. |
| d. If the risk assessment has been reviewed, is it still adequate? |
The Is reviewed assessment still adequate? value on the Device Information record of the Device-type Case Product. |
| d. Results of the assessment |
The Risk Assessment Results from the Case Product. |
| e. IMDRF 'Cause Investigation' terms and codes (Annex B, C, D) |
If the Device Type Code of the Device-type Case Product is Type of Investigation, Investigation Finding, or Investigation Conclusion, Vault exports an IMDRF code using the following priority:
- Level 3
- Level 2
- Level 1
|
| f. IMDRF Component codes (Annex G) |
If the Device Type Code of the Device-type Case Product is Medical Device Component, Vault exports an IMDRF code using the following priority:
- Level 3
- Level 2
- Level 1
|
| g. Description of remedial action/corrective action/preventive action/field safety corrective action (FSCA) |
The Corrective/Preventative Action text on the Manufacturer Analysis of the Device-type Case Product. |
| h. Time schedule for the implementation of the identified actions |
The Corrective/Preventative Action Schedule text on the Manufacturer Analysis of the Device-type Case Product. |
| i. Final comments from the manufacturer on cause investigation and conclusion |
The Investigation Summary and Conclusion text on the Manufacturer Analysis of the Device-type Case Product. |
4.3 Similar serious incidents (for Final serious incidents)
This includes additional information about similar incidents and the codes and terms used to identify them.
4.3.1 Use of IMDRF terms and codes for identifying similar incidents
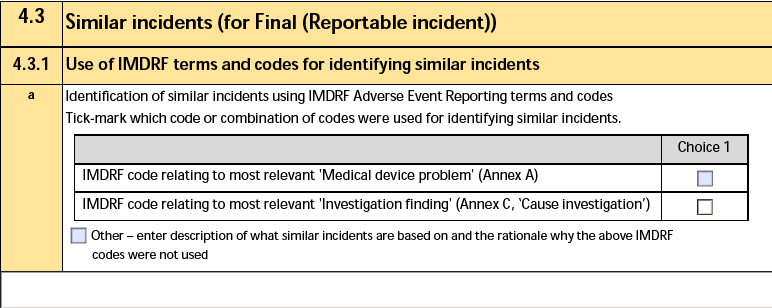
The form fields include:
| MIR Form Field |
Data Source |
| a. Identification of similar serious incidents using IMDRF Adverse Event Reporting terms and codes |
The IMDRF Similar Incident Identification on the Device Incident Report of the Device-type Case Product. Depending on the selected values, Vault may select one or both of the checkboxes for Medical device problem and Investigation finding.
When the IMDRF Similar Incident Identification (Other) field includes a value, Vault selects only the Other checkbox and exports the IMDRF Similar Incident (Other) text. |
4.3.2 Use of in-house terms/codes for identifying similar incidents
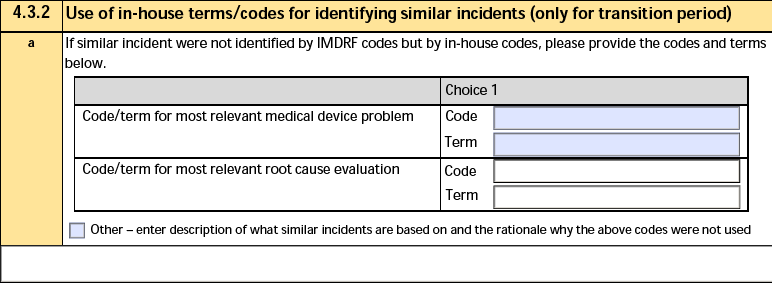
The form fields include:
| MIR Form Field |
Data Source |
| a. If similar serious incident were not identified by IMDRF codes but by in-house codes, please provide the codes and terms below. |
Vault does not populate this field. |
4.3.3 Number of similar serious incidents and devices on the market
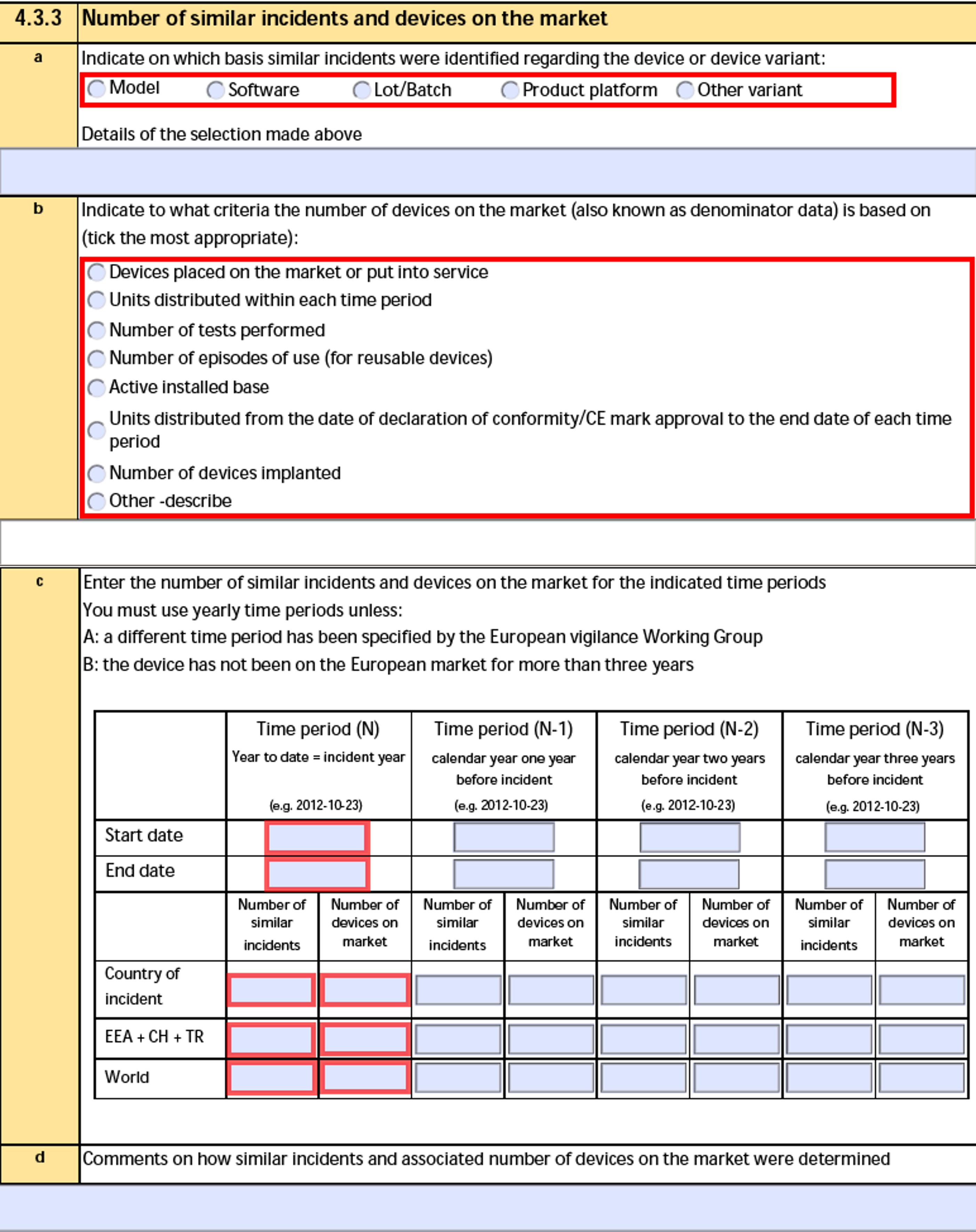
The form fields include:
| MIR Form Field |
Data Source |
| a. Indicate on which basis similar serious incidents were identified regarding the device or device variant: |
The Similar Incident Basis selection and the Similar Incident Basis Details text on the Device Incident Report of the Device-type Case Product. |
| b. Indicate on which criteria the number of devices on the market (also known as denominator data) is based on |
The Devices Marketed Criteria on the Device Incident Report of the Device-type Case Product.
When the Devices Marketed Criteria is Other, Vault also exports the Devices Marketed Criteria (Other) text. |
| c. Enter the number of similar serious incidents (SI) and devices on the market for the indicated time periods |
For each time period and region, Vault exports the Start Date and End Date from each Case Product Similar Incident record. |
| d. Comments on how similar (serious) incidents and associated number of devices on the market were determined |
The Similar Incident Comments text on the Device Incident Report of the Device-type Case Product. |

Vault populates the Device Comments on the Case Product.
Coded summary of report
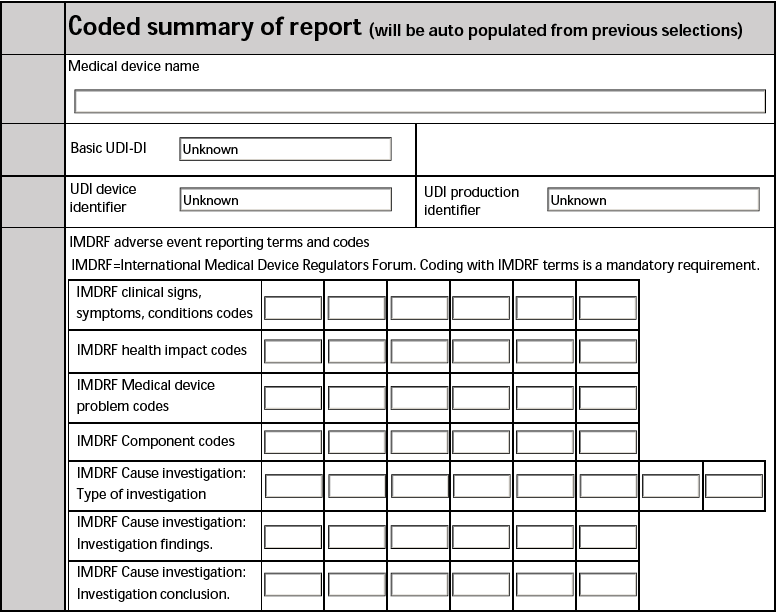
In the Coded summary of report section, Vault populates a summary of the Case using the information from the rest of the form.






















