About the Feature
Support for Combination Products was added in 20R2. Vaults created in 20R2 or later include this functionality by default, while vaults created in 20R1 or earlier must have the configuration upgrades described on this page to enable this feature.
Once you enable this feature, Manage Combination Products describes how to set up and use Combination Products.
Vaccine Combination Products
You must perform additional configuration to use combination products with a vaccine constituent. The vaccine feature enablement instructions provide more information.
Mandatory Configuration
The following configuration is required to enable Combination Products.
Add the Combination Product Page Layout
- In Admin, go to Configuration > Objects > Product.
- On the Page Layouts tab, open the Combination Product Detail Page Layout. If this layout does not exist, create a new layout with this name for the Combination Product object type.
- Update the Details section to match the following fields:
- Organization
- Product Name
- Combination Type
- Abbreviation
- Generic Name
- Manufacturer
- Core Datasheet
- Add a Product Constituents Related Object section with the following details:
- Related Object: Product Constituent
- Section Label: Product Constituents
- Creation Option: Create record in new page
- Columns:
- Name
- Product Type
- Constituent Product
- Add a Product Registrations Related Object section with the following settings:
- Related Object: Registration
- Section Label: Product Registrations
- Creation Option: Create record in new page
- Columns:
- Name
- Registration Type
- Product Constituent
- PMOA
- Country
- Agency
Update the Case Page Layout
- In Admin, go to Configuration > Objects > Case.
- On the Page Layouts tab, open the Case Page Layout.
- Add a Device Details section with the following fields:
- Device Report Type
- Malfunction-Only
- Device Follow-Up Type
- Remedial Action
- Remedial Action - Other
Update the Device Case Product Page Layout
- Go to Configuration > Objects > Case Product.
- On the Page Layouts tab, open the Device Detail Page Layout.
- Edit the Device Information section to add the following fields:
- Device Evaluated
- Manufacture Date (combined control)
- Device Usage Type
- Device Age (combined control)
- Add a Device Problem and Evaluation Codes section with the following fields:
- Device Problem
- Evaluation Method
- Evaluation Result
- Evaluation Conclusion
Update the Submission Page Layout
- Go to Configuration > Objects > Transmission.
- On the Page Layouts tab, open the Submission Detail Page Layout.
- Add the FDA Report Type field to the Details section.
Configure Tabs
- Go to Configuration > Tabs.
- Expand Business Admin (Quick Access) and select Product Library.
- Add a new Product Library tab with all of the original object types, with the addition of Combination Products.
- Remove the previously used Product Library tab.
Update Permissions
You must update permission sets to make the Combination Products feature available to users.
Great Read Access to Combination Product Components
In Admin, go to Users & Groups > Permission Sets and update the following permission sets:
- Administration Actions
- Case Distribution Actions
- Case Entry Actions
- Case Intake Actions
- Safety Operations Actions
- Safety Writer
- Submission Actions
Ensure each of the above permission sets have Read access to the following components:
| Combination Product object type under Product | |
| Product Constituent object | |
| Each field within the Product Constituent object | |
| Device Follow-Up Type object type under Controlled Vocabulary | |
| Device Usage Type object type under Controlled Vocabulary | |
| Remedial Action object type under Controlled Vocabulary | |
| Device Problem object type under Device Code | |
| Evaluation Conclusion object type under Device Code | |
| Evaluation Method object type under Device Code | |
| Evaluation Result object type under Device Code |
Grant Full Combination Product Management Access to Admins
For the Administration Action permission set, grant Create, Edit, and Delete permissions to the following components:
| Combination Product object type under Product | |
| Product Constituent object |
Recommended Configuration
The following configuration is optional but recommended for the best user experience.
Add Combination Products to the Organization Page Layout for Sponsors
- Go to Configuration > Objects > Organization.
- On the Page Layouts tab, open the Sponsor Detail Page Layout.
- Edit the Product related object section to add the following filter:
Product Type|is not equal to|Combination Product - Add a Combination Products related object section with the following filter:
Product Type|is equal to|Combination Product - In the Combination Products section, add the following columns:
- Product Name
- Combination Type
Add Combination Products to Product Page Layouts
- Go to Configuration > Objects > Product.
- On the Page Layouts tab, open each of the following layouts:
- Device Detail Page Layout
- Biologic Page Layout
- Drug Page Layout Make the following changes to each of these layouts.
- Edit the Registrations section to add the PMOA field.
- Add a new related object section with the following details:
- Related Object:
Product Constituent - Section Label:
Combination Products - Columns:
- Name
- Combination Product
- Related Object:
Update the Product Registration Page Layout
- In Admin, go to Configuration > Objects > Product Registration.
- On the Page Layouts tab, open the Product Registration Detail Page Layout.
- Edit the Details section to add the following fields:
- Product Constituent
- Product Type
- PMOA
Update Case Product Page Layouts
- In Admin, go to Configuration > Objects > Case Product.
- On the Page Layouts tab, open each of the following layouts:
- Device Detail Page Layout
- Biologic Page Layout
- Drug Page Layout Make the following changes to each of these layouts.
- Edit the Details section to add the following fields:
- Combination Product
- Combination Product Registration
Optional Template Updates for Combination Products
The following configuration was a 20R3 Template Revision and is included by default in vaults configured in 20R3 or later.
For legacy vaults, this configuration is optional but may improve user experience.
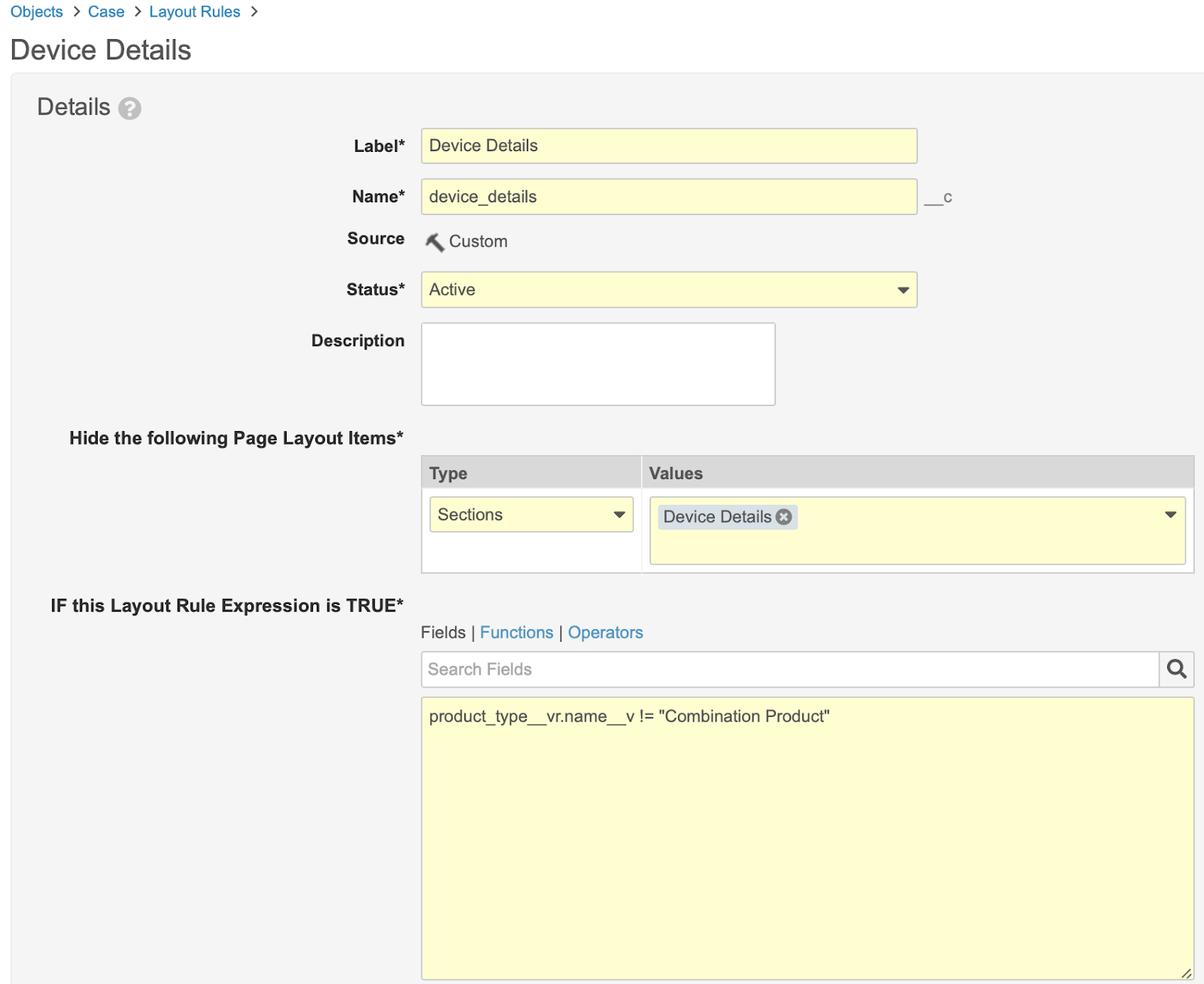
Update the Case Page Layout Rules with Device Details
- In Admin, go to Configuration > Objects > Case > Page Layouts > Case Page Layout > Layout Rules.
- Select Create.
- Create the layout rule with the following details:
- Label: Device Details
- Name:
device_details__c - Hide the following Page Layout Items:
- Type: Sections
- Values: Device Details
- IF this Layout Rule Expression is TRUE:
product_type__vr.name__v != "Combination Product"
- Select Save.
Update the Constituent Product Field on Product Constituent Object
- In Admin, go to Configuration > Objects > Product Constituent > Fields > Constituent Product.
- Select Edit.
- Update the Criteria VQL field to:
organization_v = this.organizationv AND object_typevr.api_namev != 'combination_product_v' - Select Save.

